
Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken by mouth or inhaled.
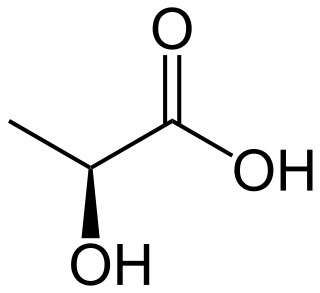
Lactic acidosis is a medical condition characterized by a build-up of lactate in the body, with formation of an excessively low pH in the bloodstream. It is a form of metabolic acidosis, in which excessive acid accumulates due to a problem with the body's oxidative metabolism.

Leigh syndrome is an inherited neurometabolic disorder that affects the central nervous system. It is named after Archibald Denis Leigh, a British neuropsychiatrist who first described the condition in 1951. Normal levels of thiamine, thiamine monophosphate, and thiamine diphosphate are commonly found but there is a reduced or absent level of thiamine triphosphate. This is thought to be caused by a blockage in the enzyme thiamine-diphosphate kinase, and therefore treatment in some patients would be to take thiamine triphosphate daily.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

Lamivudine, commonly called 3TC, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is also used to treat chronic hepatitis B when other options are not possible. It is effective against both HIV-1 and HIV-2. It is typically used in combination with other antiretrovirals such as zidovudine and abacavir. Lamivudine may be included as part of post-exposure prevention in those who have been potentially exposed to HIV. Lamivudine is taken by mouth as a liquid or tablet.
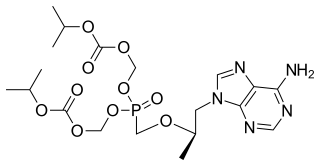
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder.

The Cori cycle, named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate, produced by anaerobic glycolysis in muscles, is transported to the liver and converted to glucose, which then returns to the muscles and is cyclically metabolized back to lactate.

Entecavir (ETV), sold under the brand name Baraclude, is an antiviral medication used in the treatment of hepatitis B virus (HBV) infection. In those with both HIV/AIDS and HBV antiretroviral medication should also be used. Entecavir is taken by mouth as a tablet or solution.

Nucleoside analogues are nucleosides which contain a nucleic acid analogue and a sugar. Nucleotide analogs are nucleotides which contain a nucleic acid analogue, a sugar, and a phosphate group with one to three phosphates.
Dichloroacetic acid (DCA), sometimes called bichloroacetic acid (BCA), is the chemical compound with formula CHCl
2COOH. It is an acid, an analogue of acetic acid, in which 2 of the 3 hydrogen atoms of the methyl group have been replaced by chlorine atoms. Like the other chloroacetic acids, it has various practical applications. The salts and esters of dichloroacetic acid are called dichloroacetates. Salts of DCA have been studied as potential drugs because they inhibit the enzyme pyruvate dehydrogenase kinase.

Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is one of the family of mitochondrial diseases, which also include MIDD (maternally inherited diabetes and deafness, MERRF syndrome, and Leber's hereditary optic neuropathy. It was first characterized under this name in 1984. A feature of these diseases is that they are caused by defects in the mitochondrial genome which is inherited purely from the female parent. The most common MELAS mutation is mitochondrial mutation, mtDNA, referred to as m.3243A>G.

Succinyl coenzyme A synthetase is an enzyme that catalyzes the reversible reaction of succinyl-CoA to succinate. The enzyme facilitates the coupling of this reaction to the formation of a nucleoside triphosphate molecule from an inorganic phosphate molecule and a nucleoside diphosphate molecule. It plays a key role as one of the catalysts involved in the citric acid cycle, a central pathway in cellular metabolism, and it is located within the mitochondrial matrix of a cell.

Lamivudine/zidovudine, sold under the brand name Combivir among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains two antiretroviral medications, lamivudine and zidovudine. It is used together with other antiretrovirals. It is taken by mouth twice a day.
Mitochondrial toxicity is a condition in which the mitochondria of a body's cells become damaged or decline significantly in number; it occurs as a side effect of certain antiretroviral drugs used to treat human immunodeficiency virus, or HIV.

Cytochrome b-c1 complex subunit 2, mitochondrial (UQCRC2), also known as QCR2, UQCR2, or MC3DN5 is a protein that in humans is encoded by the UQCRC2 gene. The product of UQCRC2 is a subunit of the respiratory chain protein Ubiquinol Cytochrome c Reductase, which consists of the products of one mitochondrially encoded gene, MTCYTB and ten nuclear genes: UQCRC1, UQCRC2, Cytochrome c1, UQCRFS1, UQCRB, "11kDa protein", UQCRH, Rieske Protein presequence, "cyt. c1 associated protein", and "Rieske-associated protein." Defects in UQCRC2 are associated with mitochondrial complex III deficiency, nuclear, type 5.

High anion gap metabolic acidosis is a form of metabolic acidosis characterized by a high anion gap. Metabolic acidosis occurs when the body produces too much acid, or when the kidneys are not removing enough acid from the body. Several types of metabolic acidosis occur, grouped by their influence on the anion gap.
Mitochondrially encoded tRNA phenylalanine also known as MT-TF is a transfer RNA which in humans is encoded by the mitochondrial MT-TF gene.

The organic anion transporter 1 (OAT1) also known as solute carrier family 22 member 6 (SLC22A6) is a protein that in humans is encoded by the SLC22A6 gene. It is a member of the organic anion transporter (OAT) family of proteins. OAT1 is a transmembrane protein that is expressed in the brain, the placenta, the eyes, smooth muscles, and the basolateral membrane of proximal tubular cells of the kidneys. It plays a central role in renal organic anion transport. Along with OAT3, OAT1 mediates the uptake of a wide range of relatively small and hydrophilic organic anions from plasma into the cytoplasm of the proximal tubular cells of the kidneys. From there, these substrates are transported into the lumen of the nephrons of the kidneys for excretion. OAT1 homologs have been identified in rats, mice, rabbits, pigs, flounders, and nematodes.

Mitochondrial DNA depletion syndrome, or Alper's disease, is any of a group of autosomal recessive disorders that cause a significant drop in mitochondrial DNA in affected tissues. Symptoms can be any combination of myopathic, hepatopathic, or encephalomyopathic. These syndromes affect tissue in the muscle, liver, or both the muscle and brain, respectively. The condition is typically fatal in infancy and early childhood, though some have survived to their teenage years with the myopathic variant and some have survived into adulthood with the SUCLA2 encephalomyopathic variant. There is currently no curative treatment for any form of MDDS, though some preliminary treatments have shown a reduction in symptoms.

Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.

















