
Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases.

Flavin is the common name for a group of organic compounds based on pteridine, formed by the tricyclic heterocycle isoalloxazine. The biochemical source is the vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide, a phosphorylated form of riboflavin. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins.

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.
A biogenic amine is a biogenic substance with one or more amine groups. They are basic nitrogenous compounds formed mainly by decarboxylation of amino acids or by amination and transamination of aldehydes and ketones. Biogenic amines are organic bases with low molecular weight and are synthesized by microbial, vegetable and animal metabolisms. In food and beverages they are formed by the enzymes of raw material or are generated by microbial decarboxylation of amino acids.
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membrane of synaptic vesicles of presynaptic neurons. It acts to transport monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses as chemical messages to postsynaptic neurons. VMATs utilize a proton gradient generated by V-ATPases in vesicle membranes to power monoamine import.

In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex.

Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin: the flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN).

Iproniazid is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class. It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until fairly recently.

Monoamine oxidase A, also known as MAO-A, is an enzyme that in humans is encoded by the MAOA gene. This gene is one of two neighboring gene family members that encode mitochondrial enzymes which catalyze the oxidative deamination of amines, such as dopamine, norepinephrine, and serotonin. A mutation of this gene results in Brunner syndrome. This gene has also been associated with a variety of other psychiatric disorders, including antisocial behavior. Alternatively spliced transcript variants encoding multiple isoforms have been observed.
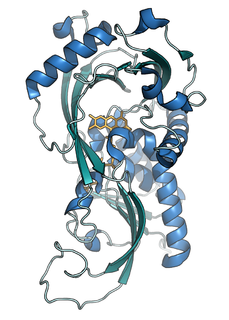
D-amino acid oxidase is an enzyme with the function on a molecular level to oxidize D-amino acids to the corresponding α-keto acids, producing ammonia and hydrogen peroxide. This results in a number of physiological effects in various systems, most notably the brain. The enzyme is most active toward neutral D-amino acids, and not active toward acidic D-amino acids. One of its most important targets in mammals is D-Serine in the central nervous system. By targeting this and other D-amino acids in vertebrates, DAAO is important in detoxification. The role in microorganisms is slightly different, breaking down D-amino acids to generate energy.
Oxidative deamination is a form of deamination that generates α-keto acids and other oxidized products from amine-containing compounds, and occurs primarily in the liver. Oxidative deamination is stereospecific, meaning it contains different stereoisomers as reactants and products; this process is either catalyzed by L or D- amino acid oxidase and L-amino acid oxidase is present only in the liver and kidney. Oxidative deamination is an important step in the catabolism of amino acids, generating a more metabolizable form of the amino acid, and also generating ammonia as a toxic byproduct. The ammonia generated in this process can then be neutralized into urea via the urea cycle.

Monoamine oxidase B, also known as MAOB, is an enzyme that in humans is encoded by the MAOB gene.

Amine oxidase (copper-containing) (AOC) (EC 1.4.3.21 and EC 1.4.3.22; formerly EC 1.4.3.6) is a family of amine oxidase enzymes which includes both primary-amine oxidase and diamine oxidase; these enzymes catalyze the oxidation of a wide range of biogenic amines including many neurotransmitters, histamine and xenobiotic amines. They act as a disulphide-linked homodimer. They catalyse the oxidation of primary amines to aldehydes, with the subsequent release of ammonia and hydrogen peroxide, which requires one copper ion per subunit and topaquinone as cofactor:
In enzymology, an L-amino acid oxidase (LAAO) (EC 1.4.3.2) is an enzyme that catalyzes the chemical reaction

Amine oxidase, copper containing 3, also known as vascular adhesion protein (VAP-1) and HPAO is an enzyme that in humans is encoded by the AOC3 gene on chromosome 17. This protein is a member of the semicarbazide-sensitive amine oxidase family of enzymes and is associated with many vascular diseases.

Spermine oxidase is an enzyme that in humans is encoded by the SMOX gene.

Primary-amine oxidase, also known as semicarbazide-sensitive amine oxidase (SSAO), is an enzyme (EC 1.4.3.21) with the systematic name primary-amine:oxygen oxidoreductase (deaminating). This enzyme catalyses the following chemical reaction
Polyamine oxidase (propane-1,3-diamine-forming) (EC 1.5.3.14, MPAO, maize PAO) is an enzyme with systematic name spermidine:oxygen oxidoreductase (propane-1,3-diamine-forming). This enzyme catalyses the following chemical reaction














