Related Research Articles

Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.

Wilson's disease is a genetic disorder in which excess copper builds up in the body. Symptoms are typically related to the brain and liver. Liver-related symptoms include vomiting, weakness, fluid build-up in the abdomen, swelling of the legs, yellowish skin, and itchiness. Brain-related symptoms include tremors, muscle stiffness, trouble in speaking, personality changes, anxiety, and psychosis.

Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection period, people often have mild or no symptoms. Early symptoms can include fever, dark urine, abdominal pain, and yellow tinged skin. The virus persists in the liver, becoming chronic, in about 70% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.
Liver function tests, also referred to as a hepatic panel, are groups of blood tests that provide information about the state of a patient's liver. These tests include prothrombin time (PT/INR), activated partial thromboplastin time (aPTT), albumin, bilirubin, and others. The liver transaminases aspartate transaminase and alanine transaminase are useful biomarkers of liver injury in a patient with some degree of intact liver function.

Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis. HCC is the third leading cause of cancer-related deaths worldwide.

Alcoholic liver disease (ALD), also called alcohol-related liver disease (ARLD), is a term that encompasses the liver manifestations of alcohol overconsumption, including fatty liver, alcoholic hepatitis, and chronic hepatitis with liver fibrosis or cirrhosis.

Gamma-glutamyltransferase is a transferase that catalyzes the transfer of gamma-glutamyl functional groups from molecules such as glutathione to an acceptor that may be an amino acid, a peptide or water. GGT plays a key role in the gamma-glutamyl cycle, a pathway for the synthesis and degradation of glutathione as well as drug and xenobiotic detoxification. Other lines of evidence indicate that GGT can also exert a pro-oxidant role, with regulatory effects at various levels in cellular signal transduction and cellular pathophysiology. This transferase is found in many tissues, the most notable one being the liver, and has significance in medicine as a diagnostic marker.

Hepatotoxicity implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval.

Hyperammonemia is a metabolic disturbance characterised by an excess of ammonia in the blood. It is a dangerous condition that may lead to brain injury and death. It may be primary or secondary.
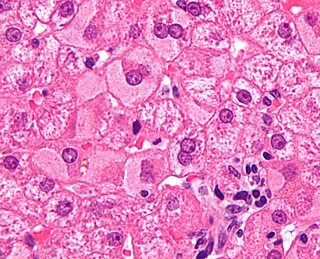
Viral hepatitis is liver inflammation due to a viral infection. It may present in acute form as a recent infection with relatively rapid onset, or in chronic form, typically progressing from a long-lasting asymptomatic condition up to a decompensated hepatic disease and hepatocellular carcinoma (HCC).

Alcoholic hepatitis is hepatitis due to excessive intake of alcohol. Patients typically have a history of at least 10 years of heavy alcohol intake, typically 8–10 drinks per day. It is usually found in association with fatty liver, an early stage of alcoholic liver disease, and may contribute to the progression of fibrosis, leading to cirrhosis. Symptoms may present acutely after a large amount of alcoholic intake in a short time period, or after years of excess alcohol intake. Signs and symptoms of alcoholic hepatitis include jaundice, ascites, fatigue and hepatic encephalopathy. Mild cases are self-limiting, but severe cases have a high risk of death. Severe cases may be treated with glucocorticoids. The condition often comes on suddenly and may progress in severity very rapidly.

Autoimmune hepatitis, formerly known as lupoid hepatitis, plasma cell hepatitis, or autoimmune chronic active hepatitis, is a chronic, autoimmune disease of the liver that occurs when the body's immune system attacks liver cells, causing the liver to be inflamed. Common initial symptoms may include fatigue, nausea, muscle aches, or weight loss or signs of acute liver inflammation including fever, jaundice, and right upper quadrant abdominal pain. Individuals with autoimmune hepatitis often have no initial symptoms and the disease may be detected by abnormal liver function tests and increased protein levels during routine bloodwork or the observation of an abnormal-looking liver during abdominal surgery.

Acute liver failure is the appearance of severe complications rapidly after the first signs of liver disease, and indicates that the liver has sustained severe damage. The complications are hepatic encephalopathy and impaired protein synthesis. The 1993 classification defines hyperacute as within 1 week, acute as 8–28 days, and subacute as 4–12 weeks; both the speed with which the disease develops and the underlying cause strongly affect outcomes.
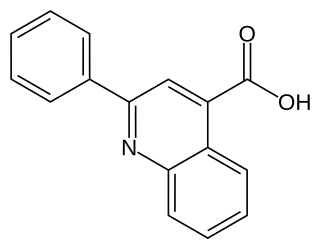
Cinchophen is an analgesic drug that was first produced by Doebner & Gieskel in 1887, it was commercially introduced in 1908 as a treatment for gout. This drug is still used, in combination with Prednisolone, by veterinarians to treat arthritis in animals. It can be prepared starting from anilin, benzaldehyde and pyruvic acid in absolute ethanol. Use of this drug in humans ceased in the 1930s due to the discovery that cinchophen can cause serious liver damage. There is some evidence that it stimulates C-Fos.

Imipenem/cilastatin, sold under the brand name Primaxin among others, is an antibiotic useful for the treatment of a number of bacterial infections. It is made from a combination of imipenem and cilastatin. Specifically it is used for pneumonia, sepsis, endocarditis, joint infections, intra-abdominal infections, and urinary tract infections. It is given by injection into a vein or muscle.
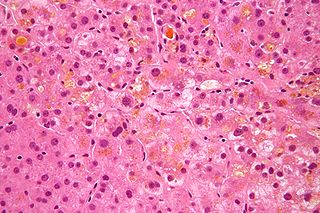
Cholestasis is a condition where the flow of bile from the liver to the duodenum is impaired. The two basic distinctions are:
Chronic liver disease in the clinical context is a disease process of the liver that involves a process of progressive destruction and regeneration of the liver parenchyma leading to fibrosis and cirrhosis. "Chronic liver disease" refers to disease of the liver which lasts over a period of six months. It consists of a wide range of liver pathologies which include inflammation, liver cirrhosis, and hepatocellular carcinoma. The entire spectrum need not be experienced.
In medicine, the presence of elevated transaminases, commonly the transaminases alanine transaminase (ALT) and aspartate transaminase (AST), may be an indicator of liver dysfunction. Other terms include transaminasemia, transaminitis, and elevatedliver enzymes. Normal ranges for both ALT and AST vary by gender, age, and geography and are roughly 8-40 U/L. Mild transaminesemia refers to levels up to 250 U/L. Drug-induced increases such as that found with the use of anti-tuberculosis agents such as isoniazid are limited typically to below 100 U/L for either ALT or AST. Muscle sources of the enzymes, such as intense exercise, are unrelated to liver function and can markedly increase AST and ALT. Cirrhosis of the liver or fulminant liver failure secondary to hepatitis commonly reach values for both ALT and AST in the >1000 U/L range; however, many people with liver disease have normal transaminases. Elevated transaminases that persist less than six months are termed "acute" in nature, and those values that persist for six months or more are termed "chronic" in nature.
The AST/ALT ratio or De Ritis ratio is the ratio between the concentrations of two enzymes, aspartate transaminase (AST) and alanine transaminase, aka alanine aminotransferase (ALT), in the blood of a human or animal. It is used as one of several liver function tests, and measured with a blood test. It is sometimes useful in medical diagnosis for elevated transaminases to differentiate between causes of liver damage, or hepatotoxicity.
Hyperbilirubinemia is a clinical condition describing an elevation of blood bilirubin level due to the inability to properly metabolise or excrete bilirubin, a product of erythrocytes breakdown. In severe cases, it is manifested as jaundice, the yellowing of tissues like skin and the sclera when excess bilirubin deposits in them. The US records 52,500 jaundice patients annually. By definition, bilirubin concentration of greater than 3 mg/ml is considered hyperbilirubinemia, following which jaundice progressively develops and becomes apparent when plasma levels reach 20 mg/ml. Rather than a disease itself, hyperbilirubinemia is indicative of multifactorial underlying disorders that trace back to deviations from regular bilirubin metabolism. Diagnosis of hyperbilirubinemia depends on physical examination, urinalysis, serum tests, medical history and imaging to identify the cause. Genetic diseases, alcohol, pregnancy and hepatitis viruses affect the likelihood of hyperbilirubinemia. Causes of hyperbilirubinemia mainly arise from the liver. These include haemolytic anaemias, enzymatic disorders, liver damage and gallstones. Hyperbilirubinemia itself is often benign. Only in extreme cases does kernicterus, a type of brain injury, occur. Therapy for adult hyperbilirubinemia targets the underlying diseases but patients with jaundice often have poor outcomes.
References
- ↑ "Flavocoxid". livertox.nlm.nih.gov. Archived from the original on 2019-08-19. Retrieved 2016-07-20.
 This article incorporates text from this source, which is in the public domain .
This article incorporates text from this source, which is in the public domain . - ↑ Levy, Robert M.; Khokhlov, Alexander; Kopenkin, Sergey; Bart, Boris; Ermolova, Tatiana; Kantemirova, Raiasa; Mazurov, Vadim; Bell, Marjorie; Caldron, Paul (2010-10-01). "Efficacy and safety of flavocoxid, a novel therapeutic, compared with naproxen: a randomized multicenter controlled trial in subjects with osteoarthritis of the knee". Advances in Therapy. 27 (10): 731–742. doi:10.1007/s12325-010-0064-z. ISSN 1865-8652. PMID 20845002. S2CID 22715679.
- ↑ "Flavocoxid Drug Record". LiverTox. United States National Library of Medicine. Archived from the original on 2019-08-19. Retrieved 2016-07-20.
- ↑ Caldwell, S. H.; Feeley, J. W.; Wieboldt, T. F.; Featherston, P. L.; Dickson, R. C. (1994-01-01). "Acute hepatitis with use of over-the-counter herbal remedies". Virginia Medical Quarterly. 121 (1): 31–33. ISSN 1052-4231. PMID 8142493.
- ↑ Morgan, Sarah L.; Baggott, Joseph E.; Moreland, Larry; Desmond, Renee; Kendrach, Angela C. (2009-10-01). "The safety of flavocoxid, a medical food, in the dietary management of knee osteoarthritis". Journal of Medicinal Food. 12 (5): 1143–1148. doi:10.1089/jmf.2008.0244. ISSN 1557-7600. PMC 2784890 . PMID 19857081.