
Photoluminescence is light emission from any form of matter after the absorption of photons. It is one of many forms of luminescence and is initiated by photoexcitation, hence the prefix photo-. Following excitation, various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductors up to milliseconds for phosphoresence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours.

Cathodoluminescence is an optical and electromagnetic phenomenon in which electrons impacting on a luminescent material such as a phosphor, cause the emission of photons which may have wavelengths in the visible spectrum. A familiar example is the generation of light by an electron beam scanning the phosphor-coated inner surface of the screen of a television that uses a cathode ray tube. Cathodoluminescence is the inverse of the photoelectric effect, in which electron emission is induced by irradiation with photons.

Quantum dots (QDs) are semiconductor particles a few nanometres in size, having optical and electronic properties that differ from those of larger particles as a result of quantum mechanics. They are a central topic in nanotechnology. When the quantum dots are illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission (photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band, or the transition between discrete energy states when band structure is no longer a good definition in QDs.
A quantum dot laser is a semiconductor laser that uses quantum dots as the active laser medium in its light emitting region. Due to the tight confinement of charge carriers in quantum dots, they exhibit an electronic structure similar to atoms. Lasers fabricated from such an active media exhibit device performance that is closer to gas lasers, and avoid some of the negative aspects of device performance associated with traditional semiconductor lasers based on bulk or quantum well active media. Improvements in modulation bandwidth, lasing threshold, relative intensity noise, linewidth enhancement factor and temperature insensitivity have all been observed. The quantum dot active region may also be engineered to operate at different wavelengths by varying dot size and composition. This allows quantum dot lasers to be fabricated to operate at wavelengths previously not possible using semiconductor laser technology. One challenge in the further advances with quantum dot lasers is the presence of multicarrier Auger processes which increases the nonradiative rate upon population inversion. Auger processes are intrinsic to the material but, in contrast to bulk semiconductors, they can be engineered to some degree in quantum dots at the cost of reducing the radiative rate. Another obstacle to the specific goal of electrically-pumped quantum dot lasing is the generally weak conductivity of quantum dot films.

Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. Much of the current research on this compound is focused on its nanoparticles.
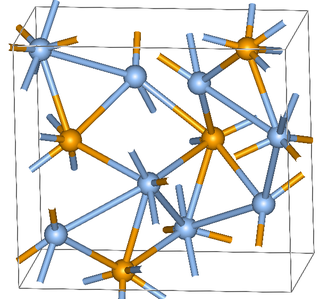
Silver selenide (Ag2Se) is the reaction product formed when selenium toning analog silver gelatine photo papers in photographic print toning. The selenium toner contains sodium selenite (Na2SeO3) as one of its active ingredients, which is the source of the selenide (Se2−) anion combining with the silver in the toning process.

A quantum dot solar cell (QDSC) is a solar cell design that uses quantum dots as the absorbing photovoltaic material. It attempts to replace bulk materials such as silicon, copper indium gallium selenide (CIGS) or cadmium telluride (CdTe). Quantum dots have bandgaps that are tunable across a wide range of energy levels by changing their size. In bulk materials, the bandgap is fixed by the choice of material(s). This property makes quantum dots attractive for multi-junction solar cells, where a variety of materials are used to improve efficiency by harvesting multiple portions of the solar spectrum.

The optical properties of carbon nanotubes are highly relevant for materials science. The way those materials interact with electromagnetic radiation is unique in many respects, as evidenced by their peculiar absorption, photoluminescence (fluorescence), and Raman spectra.

eFluor nanocrystals are a class of fluorophores made of semiconductor quantum dots. The nanocrystals can be provided as either primary amine, carboxylate, or non-functional groups on the surface, allowing conjugation to biomolecules of a researcher's choice. The nanocrystals can be conjugated to primary antibodies which are used for flow cytometry, immunohistochemistry, microarrays, in vivo imaging and microscopy.
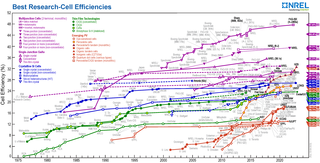
Nanocrystal solar cells are solar cells based on a substrate with a coating of nanocrystals. The nanocrystals are typically based on silicon, CdTe or CIGS and the substrates are generally silicon or various organic conductors. Quantum dot solar cells are a variant of this approach, but take advantage of quantum mechanical effects to extract further performance. Dye-sensitized solar cells are another related approach, but in this case the nano-structuring is part of the substrate.
Fluorescence intermittency, or blinking, is the phenomenon of random switching between ON (bright) and OFF (dark) states of the emitter under its continuous excitation. It is a common property of the nanoscale emitters related to the competition between the radiative and non-radiative relaxation pathways. The peculiar feature of such blinking in most cases is the power-law statistics of the ON and OFF time distributions, meaning that the measurements of the time-averaged intensity of a single emitter is not reproducible in different experiments and implying a complex dynamics of the involved process. In other words, in one experiment the emitter can blink frequently, while in another it may stay ON for almost entire length of the experiment.

Photon upconversion (UC) is a process in which the sequential absorption of two or more photons leads to the emission of light at shorter wavelength than the excitation wavelength. It is an anti-Stokes type emission. An example is the conversion of infrared light to visible light. Upconversion can take place in both organic and inorganic materials, through a number of different mechanisms. Organic molecules that can achieve photon upconversion through triplet-triplet annihilation are typically polycyclic aromatic hydrocarbons (PAHs). Inorganic materials capable of photon upconversion often contain ions of d-block or f-block elements. Examples of these ions are Ln3+, Ti2+, Ni2+, Mo3+, Re4+, Os4+, and so on.

Core–shell semiconducting nanocrystals (CSSNCs) are a class of materials which have properties intermediate between those of small, individual molecules and those of bulk, crystalline semiconductors. They are unique because of their easily modular properties, which are a result of their size. These nanocrystals are composed of a quantum dot semiconducting core material and a shell of a distinct semiconducting material. The core and the shell are typically composed of type II–VI, IV–VI, and III–V semiconductors, with configurations such as CdS/ZnS, CdSe/ZnS, CdSe/CdS, and InAs/CdSe Organically passivated quantum dots have low fluorescence quantum yield due to surface related trap states. CSSNCs address this problem because the shell increases quantum yield by passivating the surface trap states. In addition, the shell provides protection against environmental changes, photo-oxidative degradation, and provides another route for modularity. Precise control of the size, shape, and composition of both the core and the shell enable the emission wavelength to be tuned over a wider range of wavelengths than with either individual semiconductor. These materials have found applications in biological systems and optics.

DNA-functionalization of quantum dots is the attachment of strands of DNA to the surface of a quantum dot. Although quantum dots with cadmium (Cd) have some cytotoxic release, researchers have functionalized quantum dots for biocompatibility and bound them to DNA in order to combine the advantages of both materials. Quantum dots are commonly used for imaging biological systems in vitro and in vivo in animal studies due to their excellent optical properties when excited by light, while DNA has numerous bioengineering applications, including: genetic engineering, self-assembling nanostructures, protein binding, and biomarkers. The ability to visualize the chemical and biological processes of DNA allows feedback to optimize and learn about these small scale behaviors.
Quantum dots (QDs) are semiconductor nanoparticles with a size less than 10 nm. They exhibited size-dependent properties especially in the optical absorption and the photoluminescence (PL). Typically, the fluorescence emission peak of the QDs can be tuned by changing their diameters. So far, QDs were consisted of different group elements such as CdTe, CdSe, CdS in the II-VI category, InP or InAs in the III-V category, CuInS2 or AgInS2 in the I–III–VI2 category, and PbSe/PbS in the IV-VI category. These QDs are promising candidates as fluorescent labels in various biological applications such as bioimaging, biosensing and drug delivery.
Moungi Gabriel Bawendi is an American chemist of French and Tunisian descent. Born in Paris in 1961 to Hélène Baouendi and Mohammed Salah Baouendi. He is the Lester Wolfe Professor at the Massachusetts Institute of Technology. Bawendi is one of the original participants in the field of colloidal quantum dot research, and among the most cited chemists of the last decade. He became a Clarivate Citation Laureate in 2020. He received his A.B. in 1982 from Harvard University and his Ph.D. in Chemistry in 1988 from the University of Chicago working with Karl F. Freed and Takeshi Oka. It is said that in high school he joined the soccer team for one purpose only...
Light-emitting diodes (LEDs) produce light by the recombination of electrons and electron holes in a semiconductor, a process called "electroluminescence". The wavelength of the light produced depends on the energy band gap of the semiconductors used. Since these materials have a high index of refraction, design features of the devices such as special optical coatings and die shape are required to efficiently emit light. A LED is a long-lived light source, but certain mechanisms can cause slow loss of efficiency of the device or sudden failure. The wavelength of the light emitted is a function of the band gap of the semiconductor material used; materials such as gallium arsenide, and others, with various trace doping elements, are used to produce different colors of light. Another type of LED uses a quantum dot which can have its properties and wavelength adjusted by its size. Light-emitting diodes are widely used in indicator and display functions, and white LEDs are displacing other technologies for general illumination purposes.

Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.
Silicon quantum dots are metal-free biologically compatible quantum dots with photoluminescence emission maxima that are tunable through the visible to near-infrared spectral regions. These quantum dots have unique properties arising from their indirect band gap, including long-lived luminescent excited-states and large Stokes shifts. A variety of disproportionation, pyrolysis, and solution protocols have been used to prepare silicon quantum dots, however it is important to note that some solution-based protocols for preparing luminescent silicon quantum dots actually yield carbon quantum dots instead of the reported silicon. The unique properties of silicon quantum dots lend themselves to an array of potential applications: biological imaging, luminescent solar concentrators, light emitting diodes, sensors, and lithium-ion battery anodes.

Efrat Lifshitz is an Israeli chemist at the Schulich Faculty of Chemistry and the Solid-State Institute, Technion – Israel Institute of Technology (Technion-IIT). Lifshitz's research is known for pioneering advances in developing and studying low-dimensional semiconductors by exploring the relationship between their optical properties and magnetism.














