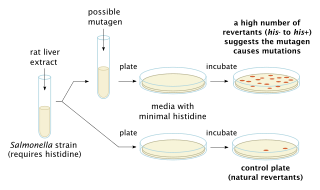
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a biological assay to assess the mutagenic potential of chemical compounds. A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer is often linked to mutation. The test serves as a quick and convenient assay to estimate the carcinogenic potential of a compound because standard carcinogen assays on mice and rats are time-consuming and expensive. However, false-positives and false-negatives are known.
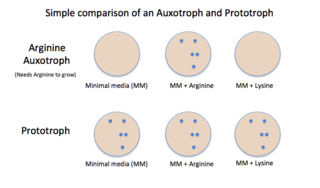
Auxotrophy is the inability of an organism to synthesize a particular organic compound required for its growth. An auxotroph is an organism that displays this characteristic; auxotrophic is the corresponding adjective. Auxotrophy is the opposite of prototrophy, which is characterized by the ability to synthesize all the compounds needed for growth.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.
Micrococcal nuclease is an endo-exonuclease that preferentially digests single-stranded nucleic acids. The rate of cleavage is 30 times greater at the 5' side of A or T than at G or C and results in the production of mononucleotides and oligonucleotides with terminal 3'-phosphates. The enzyme is also active against double-stranded DNA and RNA and all sequences will be ultimately cleaved.

Bovine pancreatic ribonuclease, also often referred to as bovine pancreatic ribonuclease A or simply RNase A, is a pancreatic ribonuclease enzyme that cleaves single-stranded RNA. Bovine pancreatic ribonuclease is one of the classic model systems of protein science. Two Nobel Prizes in Chemistry have been awarded in recognition of work on bovine pancreatic ribonuclease: in 1972, the Prize was awarded to Christian Anfinsen for his work on protein folding and to Stanford Moore and William Stein for their work on the relationship between the protein's structure and its chemical mechanism; in 1984, the Prize was awarded to Robert Bruce Merrifield for development of chemical synthesis of proteins.
In enzymology, histidinol dehydrogenase (HIS4) (HDH) (EC 1.1.1.23) is an enzyme that catalyzes the chemical reaction
Alkylglycerol monooxygenase (AGMO) is an enzyme that catalyzes the hydroxylation of alkylglycerols, a specific subclass of ether lipids. This enzyme was first described in 1964 as a pteridine-dependent ether lipid cleaving enzyme. In 2010 finally, the gene coding for alkylglycerol monooxygenase was discovered as transmembrane protein 195 (TMEM195) on chromosome 7. In analogy to the enzymes phenylalanine hydroxylase, tyrosine hydroxylase, tryptophan hydroxylase and nitric oxide synthase, alkylglycerol monooxygenase critically depends on the cofactor tetrahydrobiopterin and iron.
Arsenate reductase (glutaredoxin) (EC 1.20.4.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-disulfide reductase (glutathione) is an enzyme that catalyzes the chemical reaction
The enzyme threonine-phosphate decarboxylase (EC 4.1.1.81) catalyzes the chemical reaction
The enzyme dTDP-glucose 4,6-dehydratase (EC 4.2.1.46) catalyzes the chemical reaction

The enzyme imidazoleglycerol-phosphate dehydratase (EC 4.2.1.19) catalyzes the chemical reaction
In enzymology, a polar-amino-acid-transporting ATPase (EC 3.6.3.21) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-methylcitrate synthase (EC 2.3.3.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a glycerophospholipid acyltransferase (CoA-dependent) is an enzyme that catalyzes the chemical reaction

In enzymology, a nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a hydroxyethylthiazole kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine tele-kinase is an enzyme that catalyzes the chemical reaction

In molecular biology, cob(I)yrinic acid a,c-diamide adenosyltransferase EC 2.5.1.17 is an enzyme which catalyses the conversion of cobalamin into one of its coenzyme forms, adenosylcobalamin. Adenosylcobalamin is required as a cofactor for the activity of certain enzymes. AdoCbl contains an adenosyl moiety liganded to the cobalt ion of cobalamin via a covalent Co-C bond.
In enzymology, a ceramide phosphoethanolamine synthase is an enzyme that catalyzes the chemical reaction











