
In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base. Three main structures for the aqueous proton have garnered experimental support: the Eigen cation, which is a tetrahydrate, H3O+(H2O)3, the Zundel cation, which is a symmetric dihydrate, H+(H2O)2, and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.

Glutamic acid is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.
In chemistry, phosphorus oxoacid is a generic name for any acid whose molecule consists of atoms of phosphorus, oxygen, and hydrogen. There is a potentially infinite number of such compounds. Some of them are unstable and have not been isolated, but the derived anions and organic groups are present in stable salts and esters. The most important ones—in biology, geology, industry, and chemical research—are the phosphoric acids, whose esters and salts are the phosphates.

Isocyanic acid is a chemical compound with the structural formula HNCO, which is often written as H−N=C=O. It is a colourless, volatile and poisonous substance, with a boiling point of 23.5 °C. It is the predominant tautomer and an isomer of cyanic acid (aka. cyanol).
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.
Tetrahydroxyborate is an inorganic anion with the chemical formula [BH4O4]− or [B(OH)4]−. It contributes no colour to tetrahydroxyborate salts. It is found in the mineral hexahydroborite, Ca(B(OH)4)2 · 2 H2O, originally formulated CaB2O4 · 6 H2O. It is one of the boron oxoanions, and acts as a weak base. The systematic names are tetrahydroxyboranuide (substitutive) and tetrahydroxidoborate(1−) (additive). It can be viewed as the conjugate base of boric acid.

Croconic acid is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.

Acetylenedicarboxylic acid or butynedioic acid is an organic compound with the formula H2C4O4 or HO−C(=O)−C≡C−C(=O)−OH. It is a crystalline solid that is soluble in diethyl ether.
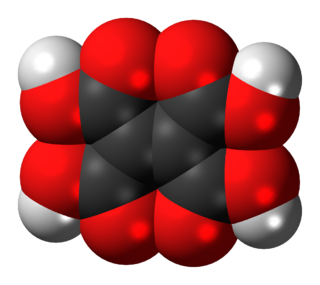
Ethylenetetracarboxylic acid is an organic compound with formula C
6H
4O
8, or (HO(OC)-)2C=C(-(CO)OH)2.

Croconate violet or 1,3-bis(dicyanomethylene)croconate is a divalent anion with chemical formula C
11N
4O2−
3 or ((N≡C−)2C=)2(C5O3)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of two oxygen atoms by dicyanomethylene groups =C(−C≡N)2. Its systematic name is 3,5-bis(dicyanomethylene)-1,2,4-trionate. The term croconate violet as a dye name specifically refers to the dipotassium salt K
2C
11N
4O
3.

In chemistry, 1,4-benzoquinonetetracarboxylic acid is an organic compound with formula C
10H
4O
10, or (C6O2)(-(CO)OH)4, which can be viewed as deriving from para-benzoquinoneC
6H
4O
2 through replacement of the four hydrogen atoms by carboxyl functional groups -(CO)OH.

A metaborate is a borate anion consisting of boron and oxygen, with empirical formula BO−2. Metaborate also refers to any salt or ester of such anion. Metaborate is one of the boron's oxyanions. Metaborates can be monomeric, oligomeric or polymeric.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.
Magnesium hydroxychloride is the traditional term for several chemical compounds of magnesium, chlorine, oxygen, and hydrogen whose general formula xMgO·yMgCl
2·zH
2O, for various values of x, y, and z; or, equivalently, Mg
x+y(OH)
2xCl
2y(H
2O)
z−x. The simple chemical formula that is often used is MgClOH, which appears in high school subject, for example.Other names for this class are magnesium chloride hydroxide, magnesium oxychloride, and basic magnesium chloride. Some of these compounds are major components of Sorel cement.

Sodium tetrahydroxyborate is a salt of with chemical formula NaH4BO4 or Na+[B(OH)4]−. It is one of several sodium borates. At room temperature it is a colorless crystalline solid.













