Related Research Articles

Entropy is a scientific concept that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication.
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applications include many problems in the fields of physics, biology, chemistry, neuroscience, computer science, information theory and sociology. Its main purpose is to clarify the properties of matter in aggregate, in terms of physical laws governing atomic motion.

Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.

The second law of thermodynamics is a physical law based on universal empirical observation concerning heat and energy interconversions. A simple statement of the law is that heat always flows spontaneously from hotter to colder regions of matter. Another statement is: "Not all heat can be converted into work in a cyclic process."

The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. The law distinguishes two principal forms of energy transfer, heat and thermodynamic work, that modify a thermodynamic system containing a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of heat and work in the system. Energy cannot be created or destroyed, but it can be transformed from one form to another. In an isolated system the sum of all forms of energy is constant.

Viscount Ilya Romanovich Prigogine was a Belgian physical chemist of Russian-Jewish origin, noted for his work on dissipative structures, complex systems, and irreversibility.

A thermodynamic potential is a scalar quantity used to represent the thermodynamic state of a system. Just as in mechanics, where potential energy is defined as capacity to do work, similarly different potentials have different meanings. The concept of thermodynamic potentials was introduced by Pierre Duhem in 1886. Josiah Willard Gibbs in his papers used the term fundamental functions.

The zeroth law of thermodynamics is one of the four principal laws of thermodynamics. It provides an independent definition of temperature without reference to entropy, which is defined in the second law. The law was established by Ralph H. Fowler in the 1930s, long after the first, second, and third laws had been widely recognized.
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of matter nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, no macroscopic change occurs.
The heat death of the universe is a hypothesis on the ultimate fate of the universe, which suggests the universe will evolve to a state of no thermodynamic free energy, and will therefore be unable to sustain processes that increase entropy. Heat death does not imply any particular absolute temperature; it only requires that temperature differences or other processes may no longer be exploited to perform work. In the language of physics, this is when the universe reaches thermodynamic equilibrium.

A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics.

Non-equilibrium thermodynamics is a branch of thermodynamics that deals with physical systems that are not in thermodynamic equilibrium but can be described in terms of macroscopic quantities that represent an extrapolation of the variables used to specify the system in thermodynamic equilibrium. Non-equilibrium thermodynamics is concerned with transport processes and with the rates of chemical reactions.
Exergy, often referred to as "available energy" or "useful work potential", is a fundamental concept in the field of thermodynamics and engineering. It plays a crucial role in understanding and quantifying the quality of energy within a system and its potential to perform useful work. Exergy analysis has widespread applications in various fields, including energy engineering, environmental science, and industrial processes.

Classical thermodynamics considers three main kinds of thermodynamic processes: (1) changes in a system, (2) cycles in a system, and (3) flow processes.
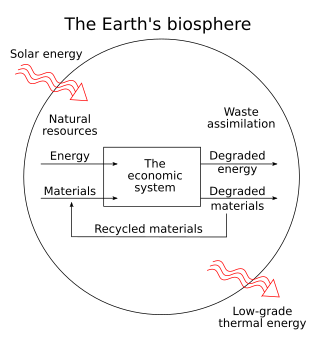
Thermoeconomics, also referred to as biophysical economics, is a school of heterodox economics that applies the laws of statistical mechanics to economic theory. Thermoeconomics can be thought of as the statistical physics of economic value and is a subfield of econophysics.
Research concerning the relationship between the thermodynamic quantity entropy and both the origin and evolution of life began around the turn of the 20th century. In 1910 American historian Henry Adams printed and distributed to university libraries and history professors the small volume A Letter to American Teachers of History proposing a theory of history based on the second law of thermodynamics and on the principle of entropy.
James J. Kay was an ecological scientist and policy-maker. He was a respected physicist best known for his theoretical work on complexity and thermodynamics.

Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.

Milivoje Kostic, is a Serbian-American thermodynamicist and professor emeritus of mechanical engineering at Northern Illinois University, Professional Engineer (PE) in Illinois, and Section First Editor-in-Chief of Thermodynamics (2015-2024) of the journal Entropy. He is an expert in energy fundamentals and applications, including nanotechnology, with emphasis on efficiency, efficient energy use and energy conservation, and environment and sustainability.
PC-SAFT is an equation of state that is based on statistical associating fluid theory (SAFT). Like other SAFT equations of state, it makes use of chain and association terms developed by Chapman, et al from perturbation theory. However, unlike earlier SAFT equations of state that used unbonded spherical particles as a reference fluid, it uses spherical particles in the context of hard chains as reference fluid for the dispersion term.
References
- ↑ "Energy: Sources, Utilization, Legislation, Sustainability, Illinois as Model State - Scienmag". Scienmag. 5 January 2016. Retrieved 26 October 2016.
- ↑ "Energy: Sources, Utilization, Legislation, Sustainability, Illinois as Model State". EurekAlert!. Retrieved 26 October 2016.
- ↑ "Energy: Sources, Utilization, Legislation, Sustainability, Illinois as Model State | Energy News". energy.tdprofiti.com. Archived from the original on 8 November 2016. Retrieved 26 October 2016.
- ↑ "Ali Mansoori - Hmolpedia". www.eoht.info. Retrieved 26 October 2016.
- ↑ "G.Ali Mansoori (www.uic.edu/~mansoori/) - Google Scholar Citations". scholar.google.com. Archived from the original on 26 October 2016. Retrieved 26 October 2016.
- ↑ Mansoori, G. A.; Carnahan, N. F.; Starling, K. E.; Jr, T. W. Leland (15 February 1971). "Equilibrium Thermodynamic Properties of the Mixture of Hard Spheres". The Journal of Chemical Physics. 54 (4): 1523–1525. Bibcode:1971JChPh..54.1523M. doi: 10.1063/1.1675048 .
- ↑ "Equilibrium Thermodynamic Properties of the Mixture of Hard Spheres - Google Scholar". scholar.google.no. Retrieved 26 October 2016.
- ↑ Mansoori, G. Ali; Araujo, Patricia Lopes Barros de; Araujo, Elmo Silvano de (6 September 2012). Diamondoid Molecules: With Applications in Biomedicine, Materials Science, Nanotechnology & Petroleum Science. World Scientific Publishing Company. ISBN 978-9814291606.
- ↑ Mansoori, G Ali (2005). Principles of Nanotechnology. doi:10.1142/5749. ISBN 978-981-256-154-1 . Retrieved 26 October 2016.