
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

In biochemistry, allosteric regulation is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.

In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses such as change in the electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.

In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ligare, which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure.
In biochemistry, an orphan receptor is a protein that has a similar structure to other identified receptors but whose endogenous ligand has not yet been identified. If a ligand for an orphan receptor is later discovered, the receptor is referred to as an "adopted orphan". Conversely, the term orphan ligand refers to a biological ligand whose cognate receptor has not yet been identified.

In the field of molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when a ligand and a target are bound to each other to form a stable complex. Knowledge of the preferred orientation in turn may be used to predict the strength of association or binding affinity between two molecules using, for example, scoring functions.
In biology, cell signaling is the process by which a cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes.

G protein-coupled receptor kinases are a family of protein kinases within the AGC group of kinases. Like all AGC kinases, GRKs use ATP to add phosphate to Serine and Threonine residues in specific locations of target proteins. In particular, GRKs phosphorylate intracellular domains of G protein-coupled receptors (GPCRs). GRKs function in tandem with arrestin proteins to regulate the sensitivity of GPCRs for stimulating downstream heterotrimeric G protein and G protein-independent signaling pathways.

P2Y receptors are a family of purinergic G protein-coupled receptors, stimulated by nucleotides such as adenosine triphosphate, adenosine diphosphate, uridine triphosphate, uridine diphosphate and UDP-glucose.To date, 8 P2Y receptors have been cloned in humans: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14.

UTOPIA is a suite of free tools for visualising and analysing bioinformatics data. Based on an ontology-driven data model, it contains applications for viewing and aligning protein sequences, rendering complex molecular structures in 3D, and for finding and using resources such as web services and data objects. There are two major components, the protein analysis suite and UTOPIA documents.

Probable G-protein coupled receptor 123 is a protein that in humans is encoded by the GPR123 gene. It is a member of the adhesion-GPCR family of receptors. Family members are normally characterized by an extended extracellular region with a variable number of protein domains coupled to a TM7 domain via a domain known as the GPCR-Autoproteolysis INducing (GAIN) domain.

G protein-coupled receptor 119 also known as GPR119 is a G protein-coupled receptor that in humans is encoded by the GPR119 gene.

GPR156, is a human gene which encodes a G protein-coupled receptor belonging to metabotropic glutamate receptor subfamily. By sequence homology, this gene was proposed as being a possible GABAB receptor subunit, however when expressed in cells alone or with other GABAB subunits, no response to GABAB ligands could be detected. In vitro studies on GPR156 constitutive activity revealed a high level of basal activation and coupling with members of the Gi/Go heterotrimeric G protein family. In 2021, an article was reported that GPR156 modulates hair cell orientation in the cochlea. Also, it was proposed that GPR156 is related to congenital hearing loss. GPR156 in complex with any of the Gi/o heterotrimers regulates the hair cell orientation. In 2024, molecular structures of G-free and Go-bound GPR156 were characterized by using cryogenic electron microscopy.
Secretin receptor family consists of secretin receptors regulated by peptide hormones from the glucagon hormone family. The family is different from adhesion G protein-coupled receptors.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.

A GPCR oligomer is a protein complex that consists of a small number of G protein-coupled receptors (GPCRs). It is held together by covalent bonds or by intermolecular forces. The subunits within this complex are called protomers, while unconnected receptors are called monomers. Receptor homomers consist of identical protomers, while heteromers consist of different protomers.

Marta Filizola is a computational biophysicist who studies membrane proteins. Filizola's research concerns drug discovery the application of methods of computational chemistry and theoretical chemistry to biochemical and biomedical problems.
Molecular Operating Environment (MOE) is a drug discovery software platform that integrates visualization, modeling and simulations, as well as methodology development, in one package. MOE scientific applications are used by biologists, medicinal chemists and computational chemists in pharmaceutical, biotechnology and academic research. MOE runs on Windows, Linux, Unix, and macOS. Main application areas in MOE include structure-based design, fragment-based design, ligand-based design, pharmacophore discovery, medicinal chemistry applications, biologics applications, structural biology and bioinformatics, protein and antibody modeling, molecular modeling and simulations, virtual screening, cheminformatics & QSAR. The Scientific Vector Language (SVL) is the built-in command, scripting and application development language of MOE.
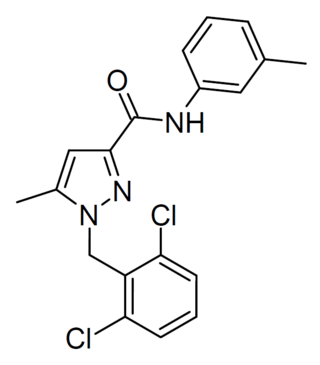
JNJ-3792165 is a chemical compound which acts as a small-molecule antagonist of the previously orphan receptor GPR139. It has been shown to modulate dopamine release in the brain via interaction with the Dopamine receptor D2, and increases the effect of morphine at the mu opioid receptor.














