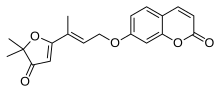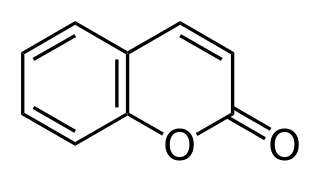
Coumarin or 2H-chromen-2-one is an aromatic organic chemical compound with formula C9H6O2. Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by a lactone-like chain −(CH)=(CH)−(C=O)−O−, forming a second six-membered heterocycle that shares two carbons with the benzene ring. It can be placed in the benzopyrone chemical class and considered as a lactone.
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellete polyketide synthesis as well as the role of protein phosphatases in cell growth.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.
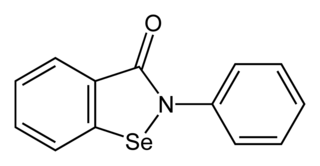
Ebselen, is a synthetic organoselenium drug molecule with anti-inflammatory, anti-oxidant and cytoprotective activity. It acts as a mimic of glutathione peroxidase and can also react with peroxynitrite. It is being investigated as a possible treatment for reperfusion injury and stroke, hearing loss and tinnitus, and bipolar disorder.

Tiazofurin is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. Tiazofurin and its analogues were under investigation for potential use in the treatment of cancer, though side effects such as pleuropericarditis and a flu-like syndrome precluded further development. They also show antiviral effects and may be reevaluated as potential options in the treatment of newly emerging viral diseases.

Aminocoumarin is a class of antibiotics that act by an inhibition of the DNA gyrase enzyme involved in the cell division in bacteria. They are derived from Streptomyces species, whose best-known representative – Streptomyces coelicolor – was completely sequenced in 2002. The aminocoumarin antibiotics include:

Monoamine oxidase B, also known as MAOB, is an enzyme that in humans is encoded by the MAOB gene.
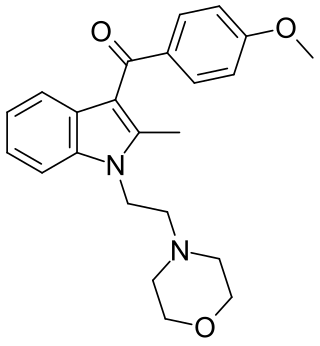
Pravadoline (WIN 48,098) is an antinflammatory and analgesic drug with an IC50 of 4.9 μM and a Ki of 2511 nM at CB1, related in structure to nonsteroidal anti-inflammatory drugs (NSAIDs) such as indometacin. It was developed in the 1980s as a new antiinflammatory and prostaglandin synthesis inhibitor, acting through inhibition of the enzyme cyclooxygenase (COX).

Serpin B9 is a protein that in humans is encoded by the SERPINB9 gene. PI9 belongs to the large superfamily of serine proteinase inhibitors (serpins), which bind to and inactivate serine proteinases. These interactions are involved in many cellular processes, including coagulation, fibrinolysis, complement fixation, matrix remodeling, and apoptosis .[supplied by OMIM]

Combretastatin A-4 is a combretastatin and a stilbenoid. It can be isolated from Combretum caffrum, the Eastern Cape South African bushwillow tree or in Combretum leprosum, the mofumbo, a species found in Brazil.

Carmofur (INN) or HCFU (1-hexylcarbamoyl-5-fluorouracil) is a pyrimidine analogue used as an antineoplastic agent. It is a derivative of fluorouracil, being a lypophilic-masked analog of 5-FU that can be administered orally.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.

JNJ-7925476 is a triple reuptake inhibitor antidepressant discovered by Johnson & Johnson, but never marketed.

Antibody-drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs.

The organic anion transporter 1 (OAT1) also known as solute carrier family 22 member 6 (SLC22A6) is a protein that in humans is encoded by the SLC22A6 gene. It is a member of the organic anion transporter (OAT) family of proteins. OAT1 is a transmembrane protein that is expressed in the brain, the placenta, the eyes, smooth muscles, and the basolateral membrane of proximal tubular cells of the kidneys. It plays a central role in renal organic anion transport. Along with OAT3, OAT1 mediates the uptake of a wide range of relatively small and hydrophilic organic anions from plasma into the cytoplasm of the proximal tubular cells of the kidneys. From there, these substrates are transported into the lumen of the nephrons of the kidneys for excretion. OAT1 homologs have been identified in rats, mice, rabbits, pigs, flounders, and nematodes.

UWA-101 is a phenethylamine derivative invented by Dr Matthew Piggott at the University of Western Australia, and researched as a potential treatment for Parkinson's disease. Its chemical structure is very similar to that of the illegal drug MDMA, the only difference being the replacement of the α-methyl group with an α-cyclopropyl group. MDMA has been found in animal studies and reported in unauthorised human self-experiments to be effective in the short-term relief of side-effects of Parkinson's disease therapy, most notably levodopa-induced dyskinesia. However the illegal status of MDMA and concerns about its potential for recreational use, neurotoxicity and potentially dangerous side effects mean that it is unlikely to be investigated for medical use in this application, and so alternative analogues were investigated.

The 3C-like protease (3CLpro) or main protease (Mpro), formally known as C30 endopeptidase or 3-chymotrypsin-like protease, is the main protease found in coronaviruses. It cleaves the coronavirus polyprotein at eleven conserved sites. It is a cysteine protease and a member of the PA clan of proteases. It has a cysteine-histidine catalytic dyad at its active site and cleaves a Gln–(Ser/Ala/Gly) peptide bond.
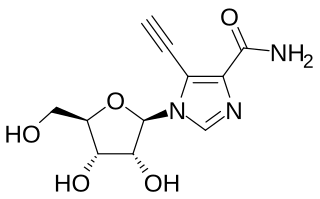
EICAR is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. It is a nucleoside derivative which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.

GS-441524 is a nucleoside analogue antiviral drug which was developed by Gilead Sciences. It is the main plasma metabolite of the antiviral prodrug remdesivir, and has a half-life of around 24 hours in human patients. Remdesivir and GS-441524 were both found to be effective in vitro against feline coronavirus strains responsible for feline infectious peritonitis (FIP), a lethal systemic disease affecting domestic cats. Remdesivir was never tested in cats, but GS-441524 has been found to be effective treatment for FIP and is widely used despite no official FDA approval due to Gilead's refusal to license this drug for veterinary use.
Katherine Seley-Radtke is an American medicinal chemist who specializes in the discovery and design of novel nucleoside or nucleotide based enzyme inhibitors that may be used to treat infections or cancer. She has authored over 90 peer-reviewed publications,is an inventor of five issued US patents, and is a Professor in the Department of Chemistry & Biochemistry at the University of Maryland, Baltimore County. Her international impact includes scientific collaborations, policy advising and diplomatic appointments in biosecurity efforts.