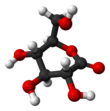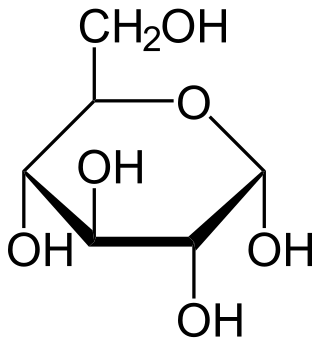
Glucose is a sugar with the molecular formula C6H12O6. Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world.
Lactones are cyclic carboxylic esters are intramolecular esters derived from hydroxy carboxylic acids. They can be saturated or unsaturated. Some contain heteroatoms replacing one or more carbon atoms of the ring.

The glucose oxidase enzyme also known as notatin is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present.
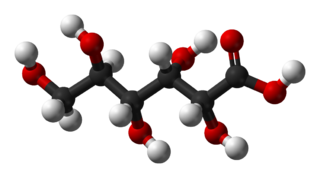
Gluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH2(CHOH)4CO2H. A white solid, it forms the gluconate anion in neutral aqueous solution. The salts of gluconic acid are known as "gluconates". Gluconic acid, gluconate salts, and gluconate esters occur widely in nature because such species arise from the oxidation of glucose. Some drugs are injected in the form of gluconates.
β-Fructofuranosidase is an enzyme that catalyzes the hydrolysis (breakdown) of the table sugar sucrose into fructose and glucose. Alternative names for β-fructofuranosidase EC 3.2.1.26 include invertase, saccharase, glucosucrase, β-fructosidase, invertin, fructosylinvertase, alkaline invertase, acid invertase, and the systematic name: β-fructofuranosidase. The resulting mixture of fructose and glucose is called inverted sugar syrup. Related to invertases are sucrases. Invertases and sucrases hydrolyze sucrose to give the same mixture of glucose and fructose. Invertase is a glycoprotein that hydrolyses (cleaves) the non-reducing terminal β-fructofuranoside residues. Invertases cleave the O-C(fructose) bond, whereas the sucrases cleave the O-C(glucose) bond. Invertase cleaves the α-1,2-glycosidic bond of sucrose.

Soured milk denotes a range of food products produced by the acidification of milk. Acidification, which gives the milk a tart taste, is achieved either through bacterial fermentation or through the addition of an acid, such as lemon juice or vinegar. The acid causes milk to coagulate and thicken, inhibiting the growth of harmful bacteria and improving the product's shelf life. It is not good for making cheese.
L-Gulonolactone oxidase is an enzyme that produces vitamin C, but is non-functional in Haplorrhini, in some bats, and in guinea pigs. It catalyzes the reaction of L-gulono-1,4-lactone with oxygen to form L-xylo-hex-3-gulonolactone (2-keto-gulono-γ-lactone) and hydrogen peroxide. It uses FAD as a cofactor. The L-xylo-hex-3-gulonolactone then converts to ascorbic acid spontaneously, without enzymatic action.
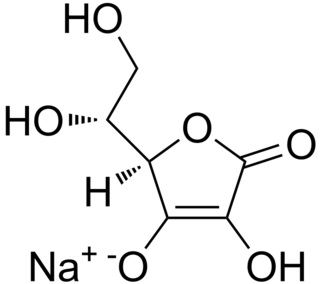
Sodium erythorbate (C6H7NaO6) is a food additive used predominantly in meats, poultry, and soft drinks. Chemically, it is the sodium salt of erythorbic acid. When used in processed meat such as hot dogs and beef sticks, it increases the rate at which nitrite reduces to nitric oxide, thus facilitating a faster cure and retaining the pink coloring. As an antioxidant structurally related to vitamin C, it helps improve flavor stability and prevents the formation of carcinogenic nitrosamines. When used as a food additive, its E number is E316. The use of erythorbic acid and sodium erythorbate as a food preservative has increased greatly since the U.S. Food and Drug Administration banned the use of sulfites as preservatives in foods intended to be eaten fresh (such as ingredients for fresh salads) and as food processors have responded to the fact that some people are allergic to sulfites. It can also be found in bologna, and is occasionally used in beverages, baked goods, and potato salad. Sodium erythorbate is produced from sugars derived from different sources, such as beets, sugarcane, and corn. Sodium erythorbate is usually produced via a fermentation process from D-glucose by Pseudomonas fluorescens bacteria. Most syntheses proceed through the 2-keto-D-gluconic acid intermediate. An urban myth claims that sodium erythorbate is made from ground earthworms; however, there is no truth to the myth. It is thought that the origin of the legend comes from the similarity of the chemical name to the words earthworm and bait.

6-Phosphogluconolactonase (EC 3.1.1.31, 6PGL, PGLS, systematic name 6-phospho-D-glucono-1,5-lactone lactonohydrolase) is a cytosolic enzyme found in all organisms that catalyzes the hydrolysis of 6-phosphogluconolactone to 6-phosphogluconic acid in the oxidative phase of the pentose phosphate pathway:

In enzymology, a glucose 1-dehydrogenase (EC 1.1.1.47) is an enzyme that catalyzes the chemical reaction
In enzymology, a glucose 1-dehydrogenase (NADP+) (EC 1.1.1.119) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-galactonolactone oxidase (EC 1.3.3.12) is an enzyme that catalyzes the chemical reaction
In enzymology, a hexose oxidase (EC 1.1.3.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a pyranose oxidase (EC 1.1.3.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a quinoprotein glucose dehydrogenase is an enzyme that catalyzes the chemical reaction
The enzyme amygdalin β-glucosidase (EC 3.2.1.117) catalyzes the following chemical reaction:
Selenium yeast is a feed additive for livestock, used to increase the selenium content in their fodder. It is a form of selenium currently approved for human consumption in the EU and Britain. Inorganic forms of selenium are used in feeds. Since these products can be patented, producers can demand premium prices. It is produced by fermenting Saccharomyces cerevisiae in a selenium-rich media.
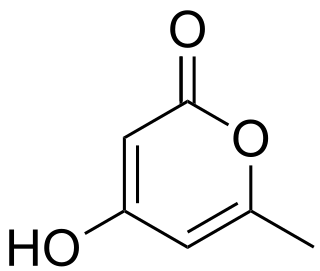
Triacetic acid lactone is an organic compound derived enzymatically from glucose. It is a light yellow solid that is soluble in organic solvents.
Aerobic fermentation or aerobic glycolysis is a metabolic process by which cells metabolize sugars via fermentation in the presence of oxygen and occurs through the repression of normal respiratory metabolism. Preference of aerobic fermentation over aerobic respiration is referred to as the Crabtree effect in yeast, and is part of the Warburg effect in tumor cells. While aerobic fermentation does not produce adenosine triphosphate (ATP) in high yield, it allows proliferating cells to convert nutrients such as glucose and glutamine more efficiently into biomass by avoiding unnecessary catabolic oxidation of such nutrients into carbon dioxide, preserving carbon-carbon bonds and promoting anabolism.

Lipase inhibitors belong to a drug class that is used as an antiobesity agent. Their mode of action is to inhibit gastric and pancreatic lipases, enzymes that play an important role in the digestion of dietary fat. Lipase inhibitors are classified in the ATC-classification system as A08AB . Numerous compounds have been either isolated from nature, semi-synthesized, or fully synthesized and then screened for their lipase inhibitory activity but the only lipase inhibitor on the market is orlistat . Lipase inhibitors have also shown anticancer activity, by inhibiting fatty acid synthase.