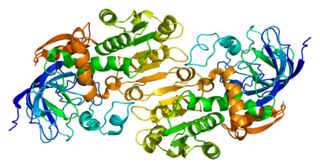
Alcohol dehydrogenases (ADH) (EC 1.1.1.1) are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. In humans and many other animals, they serve to break down alcohols that are otherwise toxic, and they also participate in the generation of useful aldehyde, ketone, or alcohol groups during the biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+.

Thermus aquaticus is a species of bacteria that can tolerate high temperatures, one of several thermophilic bacteria that belong to the Deinococcota phylum. It is the source of the heat-resistant enzyme Taq DNA polymerase, one of the most important enzymes in molecular biology because of its use in the polymerase chain reaction (PCR) DNA amplification technique.
Isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:

The Entner–Doudoroff pathway is a metabolic pathway that is most notable in Gram-negative bacteria, certain Gram-positive bacteria and archaea. Glucose is the substrate in the ED pathway and through a series of enzyme assisted chemical reactions it is catabolized into pyruvate. Entner and Doudoroff (1952) and MacGee and Doudoroff (1954) first reported the ED pathway in the bacterium Pseudomonas saccharophila. While originally thought to be just an alternative to glycolysis (EMP) and the pentose phosphate pathway (PPP), some studies now suggest that the original role of the EMP may have originally been about anabolism and repurposed over time to catabolism, meaning the ED pathway may be the older pathway. Recent studies have also shown the prevalence of the ED pathway may be more widespread than first predicted with evidence supporting the presence of the pathway in cyanobacteria, ferns, algae, mosses, and plants. Specifically, there is direct evidence that Hordeum vulgare uses the Entner–Doudoroff pathway.
Zymomonas mobilis is a Gram negative, facultative anaerobic, non-sporulating, polarly-flagellated, rod-shaped bacterium. It is the only species found in the genus Zymomonas. It has notable bioethanol-producing capabilities, which surpass yeast in some aspects. It was originally isolated from alcoholic beverages like the African palm wine, the Mexican pulque, and also as a contaminant of cider and beer in European countries.

Fructose-bisphosphate aldolase, often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Aldolase can also produce DHAP from other (3S,4R)-ketose 1-phosphates such as fructose 1-phosphate and sedoheptulose 1,7-bisphosphate. Gluconeogenesis and the Calvin cycle, which are anabolic pathways, use the reverse reaction. Glycolysis, a catabolic pathway, uses the forward reaction. Aldolase is divided into two classes by mechanism.
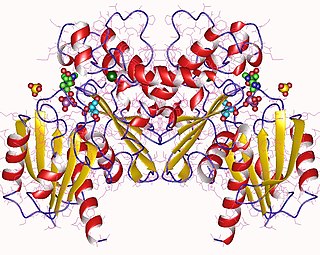
Fructokinase, also known as D-fructokinase or D-fructose (D-mannose) kinase, is an enzyme of the liver, intestine, and kidney cortex. Fructokinase is in a family of enzymes called transferases, meaning that this enzyme transfers functional groups; it is also considered a phosphotransferase since it specifically transfers a phosphate group. Fructokinase specifically catalyzes the transfer of a phosphate group from adenosine triphosphate to fructose as the initial step in its utilization. The main role of fructokinase is in carbohydrate metabolism, more specifically, sucrose and fructose metabolism. The reaction equation is as follows:
In enzymology, a succinylglutamate-semialdehyde dehydrogenase (EC 1.2.1.71) is an enzyme that catalyzes the chemical reaction
In enzymology, a D-malate dehydrogenase (decarboxylating) (EC 1.1.1.83) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-phosphoerythronate dehydogenase (EC 1.1.1.290) is an enzyme that catalyzes the chemical reaction
In enzymology, a sorbose reductase (EC 1.1.1.289) is an enzyme that catalyzes the chemical reaction
In enzymology, a 1,5-anhydro-D-fructose reductase (EC 1.1.1.263) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2,5-didehydrogluconate reductase (EC 1.1.1.274) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-hydroxy-2-methylbutyryl-CoA dehydrogenase (EC 1.1.1.178) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-oxoglutarate synthase (EC 1.2.7.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a carbon-monoxide dehydrogenase (cytochrome b-561) (EC 1.2.2.4) is an enzyme that catalyzes the chemical reaction

In enzymology, a precorrin-8X methylmutase is an enzyme that catalyzes the chemical reaction

Cobalt chelatase (EC 6.6.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, an arginine N-succinyltransferase (EC 2.3.1.109) is an enzyme that catalyzes the chemical reaction
Glucosylglycerate synthase is an enzyme with systematic name ADP-glucose:D-glycerate 2-alpha-D-glucosyltransferase. This enzyme catalyses the following chemical reaction







