
Ascorbic acid is an organic compound with formula C
6H
8O
6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves freely in water to give mildly acidic solutions. It is a mild reducing agent.

Scurvy is a disease resulting from a lack of vitamin C. Early symptoms of deficiency include weakness, fatigue, and sore arms and legs. Without treatment, decreased red blood cells, gum disease, changes to hair, and bleeding from the skin may occur. As scurvy worsens, there can be poor wound healing, personality changes, and finally death from infection or bleeding.

Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones.

Vitamin C is a water-soluble vitamin found in citrus and other fruits, berries and vegetables. It is also a generic prescription medication and in some countries is sold as a non-prescription dietary supplement. As a therapy, it is used to prevent and treat scurvy, a disease caused by vitamin C deficiency.

Pseudogenes are nonfunctional segments of DNA that resemble functional genes. Most arise as superfluous copies of functional genes, either directly by gene duplication or indirectly by reverse transcription of an mRNA transcript. Pseudogenes are usually identified when genome sequence analysis finds gene-like sequences that lack regulatory sequences needed for transcription or translation, or whose coding sequences are obviously defective due to frameshifts or premature stop codons. Pseudogenes are a type of junk DNA.
The argument from poor design, also known as the dysteleological argument, is an argument against the assumption of the existence of a creator God, based on the reasoning that any omnipotent and omnibenevolent deity or deities would not create organisms with the perceived suboptimal designs that occur in nature.
Dehydroascorbic acid (DHA) is an oxidized form of ascorbic acid. It is actively imported into the endoplasmic reticulum of cells via glucose transporters. It is trapped therein by reduction back to ascorbate by glutathione and other thiols. The (free) chemical radical semidehydroascorbic acid (SDA) also belongs to the group of oxidized ascorbic acids.

Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.
Ascorbate peroxidase (or L-ascorbate peroxidase, APX or APEX) (EC 1.11.1.11) is an enzyme that catalyzes the chemical reaction

Vitamin C megadosage is a term describing the consumption or injection of vitamin C in doses well beyond the current United States Recommended Dietary Allowance of 90 milligrams per day, and often well beyond the tolerable upper intake level of 2,000 milligrams per day. There is no scientific evidence that vitamin C megadosage helps to cure or prevent cancer, the common cold, or some other medical conditions.
Polyphenol oxidase, an enzyme involved in fruit browning, is a tetramer that contains four atoms of copper per molecule.

Pyridoxine 5′-phosphate oxidase is an enzyme, encoded by the PNPO gene, that catalyzes several reactions in the vitamin B6 metabolism pathway. Pyridoxine 5′-phosphate oxidase catalyzes the final, rate-limiting step in vitamin B6 metabolism, the biosynthesis of pyridoxal 5′-phosphate, the biologically active form of vitamin B6 which acts as an essential cofactor. Pyridoxine 5′-phosphate oxidase is a member of the enzyme class oxidases, or more specifically, oxidoreductases. These enzymes catalyze a simultaneous oxidation-reduction reaction. The substrate oxidase enzymes is hydroxlyated by one oxygen atom of molecular oxygen. Concurrently, the other oxygen atom is reduced to water. Even though molecular oxygen is the electron acceptor in these enzymes' reactions, they are unique because oxygen does not appear in the oxidized product.
In enzymology, a glucuronolactone reductase (EC 1.1.1.20) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-galactonolactone oxidase (EC 1.3.3.12) is an enzyme that catalyzes the chemical reaction
A conserved non-coding sequence (CNS) is a DNA sequence of noncoding DNA that is evolutionarily conserved. These sequences are of interest for their potential to regulate gene production.

Why Evolution is True is a popular science book by American biologist Jerry Coyne. It was published in 2009, dubbed "Darwin Year" as it marked the bicentennial of Charles Darwin and the hundred and fiftieth anniversary of the publication of his On the Origin of Species By Means of Natural Selection. Coyne examines the evidence for evolution, some of which was known to Darwin (biogeography) and some of which has emerged in recent years. The book was a New York Times bestseller, and reviewers praised the logic of Coyne's arguments and the clarity of his prose. It was reprinted as part of the Oxford Landmark Science series.
L-galactonolactone dehydrogenase (EC 1.3.2.3, galactonolactone dehydrogenase, L-galactono-gamma-lactone dehydrogenase, L-galactono-gamma-lactone:ferricytochrome-c oxidoreductase, GLDHase, GLDase) is an enzyme with systematic name L-galactono-1,4-lactone:ferricytochrome-c oxidoreductase. This enzyme catalyses the following chemical reaction
GDP-L-galactose phosphorylase is an enzyme with systematic name GDP:alpha-L-galactose 1-phosphate guanylyltransferase. This enzyme catalyses the following chemical reaction

Intravenous Ascorbic Acid, is a process that delivers soluble ascorbic acid directly into the bloodstream. It is not approved for use to treat any medical condition.
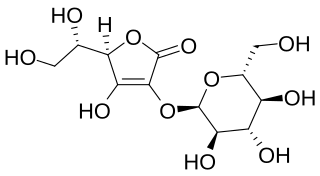
Ascorbyl glucoside (AA-2G) is an ascorbic acid derivative that contains at least one glycosyl group. Ascorbyl glucoside is commonly used in cosmetic products to administer vitamin C topically. Ascorbyl glucoside exhibits superior stability and penetration ability compared to ascorbyl phosphate salts, but the rate of its in vivo conversion to ascorbic acid is not known. Ascorbyl glucosides such as AA-2G, like many other derivatives of the ascorbic acid, show antiscorbutic effects. It is also sometimes used in skin whitening products.












