
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, and precursors to catalysts.

Marshmallow is a type of confectionery that is typically made from sugar, water and gelatin whipped to a solid-but-soft consistency. It is used as a filling in baking or normally molded into shapes and coated with corn starch. The sugar confection is inspired by a historical medicinal confection made from Althaea officinalis, the marsh-mallow plant.
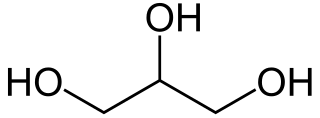
Glycerol, also called glycerine or glycerin, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. Its presence in blood can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.
Saponification is a process of cleaving esters into carboxylate salts and alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol:
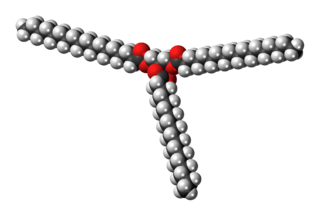
Stearin, or tristearin, or glyceryl tristearate is an odourless, white powder. It is a triglyceride derived from three units of stearic acid. Most triglycerides are derived from at least two and more commonly three different fatty acids. Like other triglycerides, stearin can crystallise in three polymorphs. For stearin, these melt at 54 (α-form), 65, and 72.5 °C (β-form).
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate Na
2SiO
3, sodium orthosilicate Na
4SiO
4, and sodium pyrosilicate Na
6Si
2O
7. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.

Rosin, also called colophony or Greek pitch, is a solid form of resin obtained from pines and some other plants, mostly conifers, produced by heating fresh liquid resin to vaporize the volatile liquid terpene components. It is semi-transparent and varies in color from yellow to black. At room temperature rosin is brittle, but it melts at stove-top temperature. It chiefly consists of various resin acids, especially abietic acid. The term colophony comes from colophonia resina, Latin for "resin from Colophon", an ancient Ionic city. It is an FDA approved food additive.

Fractional freezing is a process used in process engineering and chemistry to separate substances with different melting points. It can be done by partial melting of a solid, for example in zone refining of silicon or metals, or by partial crystallization of a liquid, as in freeze distillation, also called normal freezing or progressive freezing. The initial sample is thus fractionated.
An antifreeze is an additive which lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing-point depression for cold environments. Common antifreezes also increase the boiling point of the liquid, allowing higher coolant temperature. However, all common antifreeze additives also have lower heat capacities than water, and do reduce water's ability to act as a coolant when added to it.

In cooking, a consommé is a type of clear soup made from richly flavoured stock or broth that has been clarified, a process that uses egg whites to remove fat and sediment.
A humectant is a hygroscopic (water-absorbing) substance used to keep things moist. They are used in many products, including food, cosmetics, medicines and pesticides. When used as a food additive, a humectant has the effect of keeping moisture in the food. Humectants are sometimes used as a component of antistatic coatings for plastics.
Salting out is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed.

Kilju is the Finnish word for home made alcoholic beverage typically made of sugar, yeast, and water.

A sugar refinery is a refinery which processes raw sugar from cane or sugar extracted from beets into white refined sugar.

Fondant icing, also commonly called simply fondant, is an icing used to decorate or sculpt cakes and pastries. It is made from sugar, water, gelatin, vegetable oil or shortening, and glycerol. It does not have the texture of most icings; rolled fondant is akin to stiff clay, while poured fondant is a thick liquid. The word, in French, means 'melting,' coming from the same root as fondue and foundry.
Rocket Candy, or R-Candy, is a type of rocket propellant for model rockets made with a form of sugar as a fuel, and containing an oxidizer. The propellant can be divided into three groups of components: the fuel, the oxidizer, and the additive(s). In the past, sucrose was most commonly used as fuel. Modern formulations most commonly use sorbitol for its ease of production. The most common oxidizer is potassium nitrate (KNO3). Potassium nitrate is most commonly found in tree stump remover. Additives can be many different substances, and either act as catalysts or enhance the aesthetics of the liftoff or flight. A traditional sugar propellant formulation is typically prepared in a 65:35 (13:7) oxidizer to fuel ratio.
Degreasing, often called defatting or fat trimming, is the removal of fatty acids from an object. In culinary science, degreasing is done with the intention of reducing the fat content of a meal.

Fruit preserves are preparations of fruits whose main preserving agent is sugar and sometimes acid, often stored in glass jars and used as a condiment or spread.

A composition roller is a tool used in letterpress printing to apply ink to a bed of type in a printing press. It consists of a cylinder made of a substance known as "roller composition" or simply "composition", a mixture of hide glue and sugar, with various additives such as glycerin depending on the particular recipe. Early recipes also included gypsum plaster and tar, though these were eventually found unnecessary.












