
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. It is the main storage form of glucose in the human body.

5' AMP-activated protein kinase or AMPK or 5' adenosine monophosphate-activated protein kinase is an enzyme that plays a role in cellular energy homeostasis, largely to activate glucose and fatty acid uptake and oxidation when cellular energy is low. It belongs to a highly conserved eukaryotic protein family and its orthologues are SNF1 in yeast, and SnRK1 in plants. It consists of three proteins (subunits) that together make a functional enzyme, conserved from yeast to humans. It is expressed in a number of tissues, including the liver, brain, and skeletal muscle. In response to binding AMP and ADP, the net effect of AMPK activation is stimulation of hepatic fatty acid oxidation, ketogenesis, stimulation of skeletal muscle fatty acid oxidation and glucose uptake, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipogenesis, inhibition of adipocyte lipolysis, and modulation of insulin secretion by pancreatic β-cells.

Glycogen phosphorylase is one of the phosphorylase enzymes. Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.
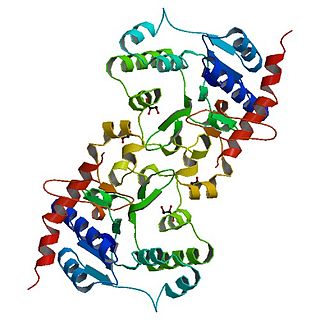
Glycogenin is an enzyme involved in converting glucose to glycogen. It acts as a primer, by polymerizing the first few glucose molecules, after which other enzymes take over. It is a homodimer of 37-kDa subunits and is classified as a glycosyltransferase.
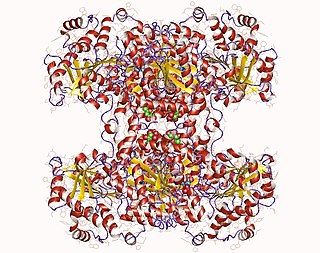
Glycogen synthase is a key enzyme in glycogenesis, the conversion of glucose into glycogen. It is a glycosyltransferase that catalyses the reaction of UDP-glucose and n to yield UDP and n+1.

The glycogen debranching enzyme, in humans, is the protein encoded by the gene AGL. This enzyme is essential for the breakdown of glycogen, which serves as a store of glucose in the body. It has separate glucosyltransferase and glucosidase activities.

Glycogen storage disease type 0 is a disease characterized by a deficiency in the glycogen synthase enzyme (GSY). Although glycogen synthase deficiency does not result in storage of extra glycogen in the liver, it is often classified as a glycogen storage disease because it is another defect of glycogen storage and can cause similar problems. There are two isoforms (types) of glycogen synthase enzyme; GSY1 in muscle and GSY2 in liver, each with a corresponding form of the disease. Mutations in the liver isoform (GSY2), causes fasting hypoglycemia, high blood ketones, increased free fatty acids and low levels of alanine and lactate. Conversely, feeding in these patients results in hyperglycemia and hyperlactatemia.

1,4-alpha-glucan-branching enzyme, also known as brancher enzyme or glycogen-branching enzyme is an enzyme that in humans is encoded by the GBE1 gene.

Acid alpha-glucosidase, also called acid maltase, is an enzyme that helps to break down glycogen in the lysosome. It is functionally similar to glycogen debranching enzyme, but is on a different chromosome, processed differently by the cell and is located in the lysosome rather than the cytosol. In humans, it is encoded by the GAA gene. Errors in this gene cause glycogen storage disease type II.

Phosphorylase kinase (PhK) is a serine/threonine-specific protein kinase which activates glycogen phosphorylase to release glucose-1-phosphate from glycogen. PhK phosphorylates glycogen phosphorylase at two serine residues, triggering a conformational shift which favors the more active glycogen phosphorylase “a” form over the less active glycogen phosphorylase b.

Myophosphorylase or glycogen phosphorylase, muscle associated (PYGM) is the muscle isoform of the enzyme glycogen phosphorylase and is encoded by the PYGM gene. This enzyme helps break down glycogen into glucose-1-phosphate, so it can be used within the muscle cell. Mutations in this gene are associated with McArdle disease, a glycogen storage disease of muscle.

UTP—glucose-1-phosphate uridylyltransferase also known as glucose-1-phosphate uridylyltransferase is an enzyme involved in carbohydrate metabolism. It synthesizes UDP-glucose from glucose-1-phosphate and UTP; i.e.,

Troponin I, fast skeletal muscle is a protein that in humans is encoded by the TNNI2 gene.

Phosphorylase b kinase gamma catalytic chain, skeletal muscle isoform is an enzyme that in humans is encoded by the PHKG1 gene.

Phosphorylase b kinase gamma catalytic chain, testis/liver isoform is an enzyme that in humans is encoded by the PHKG2 gene.

Decaprenyl-diphosphate synthase subunit 1 is an enzyme that in humans is encoded by the PDSS1 gene.

5'-AMP-activated protein kinase subunit gamma-3 is an enzyme that in humans is encoded by the PRKAG3 gene.

Phosphorylase b kinase regulatory subunit alpha, skeletal muscle isoform is an enzyme that in humans is encoded by the PHKA1 gene. It is the muscle isoform of Phosphorylase kinase (PhK).

Inborn errors of carbohydrate metabolism are inborn error of metabolism that affect the catabolism and anabolism of carbohydrates.
Glycogen phosphorylase, liver form (PYGL), also known as human liver glycogen phosphorylase (HLGP), is an enzyme that in humans is encoded by the PYGL gene on chromosome 14. This gene encodes a homodimeric protein that catalyses the cleavage of alpha-1,4-glucosidic bonds to release glucose-1-phosphate from liver glycogen stores. This protein switches from inactive phosphorylase B to active phosphorylase A by phosphorylation of serine residue 14. Activity of this enzyme is further regulated by multiple allosteric effectors and hormonal controls. Humans have three glycogen phosphorylase genes that encode distinct isozymes that are primarily expressed in liver, brain and muscle, respectively. The liver isozyme serves the glycemic demands of the body in general while the brain and muscle isozymes supply just those tissues. In glycogen storage disease type VI, also known as Hers disease, mutations in liver glycogen phosphorylase inhibit the conversion of glycogen to glucose and results in moderate hypoglycemia, mild ketosis, growth retardation and hepatomegaly. Alternative splicing results in multiple transcript variants encoding different isoforms [provided by RefSeq, Feb 2011].























