Related Research Articles

The hypothalamus is a portion of the brain that contains a number of small nuclei with a variety of functions. One of the most important functions of the hypothalamus is to link the nervous system to the endocrine system via the pituitary gland. The hypothalamus is located below the thalamus and is part of the limbic system. In the terminology of neuroanatomy, it forms the ventral part of the diencephalon. All vertebrate brains contain a hypothalamus. In humans, it is the size of an almond.

Prolactin (PRL), also known as lactotropin, is a protein best known for its role in enabling mammals to produce milk. It is influential in over 300 separate processes in various vertebrates, including humans. Prolactin is secreted from the pituitary gland in response to eating, mating, estrogen treatment, ovulation and nursing. It is secreted heavily in pulses in between these events. Prolactin plays an essential role in metabolism, regulation of the immune system and pancreatic development.
Luteinizing hormone is a hormone produced by gonadotropic cells in the anterior pituitary gland. The production of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. In females, an acute rise of LH triggers ovulation and development of the corpus luteum. In males, where LH had also been called interstitial cell–stimulating hormone (ICSH), it stimulates Leydig cell production of testosterone. It acts synergistically with follicle-stimulating hormone (FSH).

Follicle-stimulating hormone (FSH) is a gonadotropin, a glycoprotein polypeptide hormone. FSH is synthesized and secreted by the gonadotropic cells of the anterior pituitary gland and regulates the development, growth, pubertal maturation, and reproductive processes of the body. FSH and luteinizing hormone (LH) work together in the reproductive system.
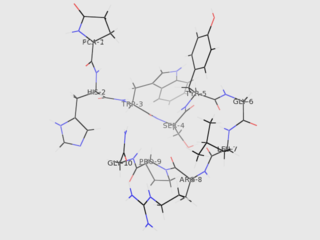
Gonadotropin-releasing hormone (GnRH) is a releasing hormone responsible for the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. GnRH is a tropic peptide hormone synthesized and released from GnRH neurons within the hypothalamus. The peptide belongs to gonadotropin-releasing hormone family. It constitutes the initial step in the hypothalamic–pituitary–gonadal axis.

The arcuate nucleus of the hypothalamus is an aggregation of neurons in the mediobasal hypothalamus, adjacent to the third ventricle and the median eminence. The arcuate nucleus includes several important and diverse populations of neurons that help mediate different neuroendocrine and physiological functions, including neuroendocrine neurons, centrally projecting neurons, and astrocytes. The populations of neurons found in the arcuate nucleus are based on the hormones they secrete or interact with and are responsible for hypothalamic function, such as regulating hormones released from the pituitary gland or secreting their own hormones. Neurons in this region are also responsible for integrating information and providing inputs to other nuclei in the hypothalamus or inputs to areas outside this region of the brain. These neurons, generated from the ventral part of the periventricular epithelium during embryonic development, locate dorsally in the hypothalamus, becoming part of the ventromedial hypothalamic region. The function of the arcuate nucleus relies on its diversity of neurons, but its central role is involved in homeostasis. The arcuate nucleus provides many physiological roles involved in feeding, metabolism, fertility, and cardiovascular regulation.
Neuroendocrine cells are cells that receive neuronal input and, as a consequence of this input, release messenger molecules (hormones) into the blood. In this way they bring about an integration between the nervous system and the endocrine system, a process known as neuroendocrine integration. An example of a neuroendocrine cell is a cell of the adrenal medulla, which releases adrenaline to the blood. The adrenal medullary cells are controlled by the sympathetic division of the autonomic nervous system. These cells are modified postganglionic neurons. Autonomic nerve fibers lead directly to them from the central nervous system. The adrenal medullary hormones are kept in vesicles much in the same way neurotransmitters are kept in neuronal vesicles. Hormonal effects can last up to ten times longer than those of neurotransmitters. Sympathetic nerve fiber impulses stimulate the release of adrenal medullary hormones. In this way the sympathetic division of the autonomic nervous system and the medullary secretions function together.
Releasing hormones and inhibiting hormones are hormones whose main purpose is to control the release of other hormones, either by stimulating or inhibiting their release. They are also called liberins and statins (respectively), or releasing factors and inhibiting factors. The principal examples are hypothalamic-pituitary hormones that can be classified from several viewpoints: they are hypothalamic hormones, they are hypophysiotropic hormones, and they are tropic hormones.
Neuroendocrinology is the branch of biology which studies the interaction between the nervous system and the endocrine system; i.e. how the brain regulates the hormonal activity in the body. The nervous and endocrine systems often act together in a process called neuroendocrine integration, to regulate the physiological processes of the human body. Neuroendocrinology arose from the recognition that the brain, especially the hypothalamus, controls secretion of pituitary gland hormones, and has subsequently expanded to investigate numerous interconnections of the endocrine and nervous systems.

The hypothalamic–pituitary–gonadal axis refers to the hypothalamus, pituitary gland, and gonadal glands as if these individual endocrine glands were a single entity. Because these glands often act in concert, physiologists and endocrinologists find it convenient and descriptive to speak of them as a single system.
The periventricular nucleus is a thin sheet of small neurons located in the wall of the third ventricle, a composite structure of the hypothalamus. It functions in analgesia.

Kisspeptins are proteins encoded by the KISS1 gene in humans. Kisspeptins are ligands of the G-protein coupled receptor, GPR54. Kiss1 was originally identified as a human metastasis suppressor gene that has the ability to suppress melanoma and breast cancer metastasis. Kisspeptin-GPR54 signaling has an important role in initiating secretion of gonadotropin-releasing hormone (GnRH) at puberty, the extent of which is an area of ongoing research. Gonadotropin-releasing hormone is released from the hypothalamus to act on the anterior pituitary triggering the release of luteinizing hormone (LH), and follicle stimulating hormone (FSH). These gonadotropic hormones lead to sexual maturation and gametogenesis. Disrupting GPR54 signaling can cause hypogonadotrophic hypogonadism in rodents and humans. The Kiss1 gene is located on chromosome 1. It is transcribed in the brain, adrenal gland, and pancreas.

Neurokinin B (NKB) belongs in the family of tachykinin peptides. Neurokinin B is implicated in a variety of human functions and pathways such as the secretion of gonadotropin-releasing hormone. Additionally, NKB is associated with pregnancy in females and maturation in young adults. Reproductive function is highly dependent on levels of both neurokinin B and also the G-protein coupled receptor ligand kisspeptin. The first NKB studies done attempted to resolve why high levels of the peptide may be implicated in pre-eclampsia during pregnancy. NKB, kisspeptin, and dynorphin together are found in the arcuate nucleus (ARC) known as the KNDy subpopulation. This subpopulation is targeted by many steroid hormones and works to form a network that feeds back to GnRH pulse generator.

The KiSS1-derived peptide receptor is a G protein-coupled receptor which binds the peptide hormone kisspeptin (metastin). Kisspeptin is encoded by the metastasis suppressor gene KISS1, which is expressed in a variety of endocrine and gonadal tissues. Activation of the kisspeptin receptor is linked to the phospholipase C and inositol trisphosphate second messenger cascades inside the cell.
Hypothalamic–pituitary hormones are hormones that are produced by the hypothalamus and pituitary gland. Although the organs in which they are produced are relatively small, the effects of these hormones cascade throughout the body. They can be classified as a hypothalamic–pituitary axis of which the adrenal (HPA), gonadal (HPG), thyroid (HPT), somatotropic (HPS), and prolactin (HPP) axes are branches.
Hypogonadotropic hypogonadism (HH), is due to problems with either the hypothalamus or pituitary gland affecting the hypothalamic-pituitary-gonadal axis. Hypothalamic disorders result from a deficiency in the release of gonadotropic releasing hormone (GnRH), while pituitary gland disorders are due to a deficiency in the release of gonadotropins from the anterior pituitary. GnRH is the central regulator in reproductive function and sexual development via the HPG axis. GnRH is released by GnRH neurons, which are hypothalamic neuroendocrine cells, into the hypophyseal portal system acting on gonadotrophs in the anterior pituitary. The release of gonadotropins, LH and FSH, act on the gonads for the development and maintenance of proper adult reproductive physiology. LH acts on Leydig cells in the male testes and theca cells in the female. FSH acts on Sertoli cells in the male and follicular cells in the female. Combined this causes the secretion of gonadal sex steroids and the initiation of folliculogenesis and spermatogenesis. The production of sex steroids forms a negative feedback loop acting on both the anterior pituitary and hypothalamus causing a pulsatile secretion of GnRH. GnRH neurons lack sex steroid receptors and mediators such as kisspeptin stimulate GnRH neurons for pulsatile secretion of GnRH.
The hormone of gonadotropins secreted by the anterior hypophyse gland effects on the gonads and play a crucial role in the process of gonadal development and function in vertebrates. In birds and mammals, luteinizinghormone (LH) regulates sex steroid production as well as ovulation, whereas follicle stimulating hormone (FSH) promotes spermatogenesis and ovarian follicle maturation. Since the isolation of gonadotropin-releasing hormone (GnRH), a hypothalamic decapeptide, from mammalian brain in the early 1970s, several other GnRHs have been identified in the brains of other vertebrates. Based on extensive studies in vertebrates, it was generally believed that GnRH is the only hypothalamic regulator of the release of pituitary gonadotropins. Some neurochemicals and peripheral hormones [e.g.gamma-aminobutyric acid (GABA), opiates, gonadal sex steroids, inhibin] can modulate gonadotropin release, but GnRH was considered to have no hypothalamic antagonist.
Pulsatile secretion is a biochemical phenomenon observed in a wide variety of cell and tissue types, in which chemical products are secreted in a regular temporal pattern. The most common cellular products observed to be released in this manner are intercellular signaling molecules such as hormones or neurotransmitters. Examples of hormones that are secreted pulsatilely include insulin, thyrotropin, TRH, gonadotropin-releasing hormone (GnRH) and growth hormone (GH). In the nervous system, pulsatility is observed in oscillatory activity from central pattern generators. In the heart, pacemakers are able to work and secrete in a pulsatile manner. A pulsatile secretion pattern is critical to the function of many hormones in order to maintain the delicate homeostatic balance necessary for essential life processes, such as development and reproduction. Variations of the concentration in a certain frequency can be critical to hormone function, as evidenced by the case of GnRH agonists, which cause functional inhibition of the receptor for GnRH due to profound downregulation in response to constant (tonic) stimulation. Pulsatility may function to sensitize target tissues to the hormone of interest and upregulate receptors, leading to improved responses. This heightened response may have served to improve the animal's fitness in its environment and promote its evolutionary retention.
Neuropeptide VF precursor, also known as pro-FMRFamide-related neuropeptide VF or RFamide-related peptide precursor, is a propeptide that in mammals is encoded by the NPVF (or RPFP) gene. The NPVF gene, and thus the propeptide, are expressed in neurons in the mediobasal hypothalamus. The propeptide is cleaved to form three other peptides, which are:
Kisspeptin, neurokinin B, and dynorphin (KNDy) neurons are neurons in the hypothalamus of the brain that are central to the hormonal control of reproduction.
References
- 1 2 Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ (August 2000). "A novel avian hypothalamic peptide inhibiting gonadotropin release". Biochemical and Biophysical Research Communications. 275 (2): 661–7. doi:10.1006/bbrc.2000.3350. PMID 10964719.
- ↑ Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M (September 2001). "Characteristics and distribution of endogenous RFamide-related peptide-1". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1540 (3): 221–32. doi: 10.1016/S0167-4889(01)00135-5 . PMID 11583817.
- ↑ Findeisen M, Rathmann D, Beck-Sickinger AG (2011). "RFamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential". Pharmaceuticals. 4 (9): 1248–1280. doi: 10.3390/ph4091248 . PMC 4058657 .
- ↑ Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R (February 2006). "Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals". Proceedings of the National Academy of Sciences of the United States of America. 103 (7): 2410–5. Bibcode:2006PNAS..103.2410K. doi: 10.1073/pnas.0511003103 . PMC 1413747 . PMID 16467147.
- 1 2 Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE (December 2009). "Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis". PLOS ONE. 4 (12): e8400. Bibcode:2009PLoSO...4.8400U. doi: 10.1371/journal.pone.0008400 . PMC 2791420 . PMID 20027225.
- 1 2 3 Smith JT, Clarke IJ (April 2010). "Gonadotropin inhibitory hormone function in mammals". Trends in Endocrinology and Metabolism. 21 (4): 255–60. doi:10.1016/j.tem.2009.11.010. PMID 20060314. S2CID 41737276.
- 1 2 Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ (October 2010). "Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate". Biology of Reproduction. 83 (4): 568–77. doi:10.1095/biolreprod.110.085407. PMC 2957156 . PMID 20574054.
- ↑ Smith JT, Young IR, Veldhuis JD, Clarke IJ (July 2012). "Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system". Endocrinology. 153 (7): 3368–75. doi:10.1210/en.2012-1088. PMC 3380300 . PMID 22549225.
- 1 2 Li X, Su J, Lei Z, Zhao Y, Jin M, Fang R, Zheng L, Jiao Y (August 2012). "Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig: cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle". Peptides. 36 (2): 176–85. doi:10.1016/j.peptides.2012.05.008. PMID 22664321. S2CID 902250.
- ↑ Calisi RM, Díaz-Muñoz SL, Wingfield JC, Bentley GE (July 2011). "Social and breeding status are associated with the expression of GnIH". Genes, Brain and Behavior. 10 (5): 557–64. doi: 10.1111/j.1601-183X.2011.00693.x . PMID 21466656.
- ↑ Calisi RM, Geraghty AC, Avila A, Kaufer D, Bentley GE, Wingfield JC (October 2016). "Patterns of hypothalamic GnIH change over the reproductive period in starlings and rats". General and Comparative Endocrinology. 237: 140–146. doi: 10.1016/j.ygcen.2016.08.015 . PMID 27591072.
- ↑ Ubuka T, Son YL, Tobari Y, Tsutsui K (2012). "Gonadotropin-inhibitory hormone action in the brain and pituitary". Frontiers in Endocrinology. 3: 148. doi: 10.3389/fendo.2012.00148 . PMC 3515997 . PMID 23233850.
- ↑ Ubuka T, Son YL, Bentley GE, Millar RP, Tsutsui K (September 2013). "Gonadotropin-inhibitory hormone (GnIH), GnIH receptor and cell signaling". General and Comparative Endocrinology. 10th International Symposium on Avian Endocrinology. 190: 10–7. doi:10.1016/j.ygcen.2013.02.030. PMID 23499786.
- ↑ Caraty A, Blomenröhr M, Vogel GM, Lomet D, Briant C, Beltramo M (May 2012). "RF9 powerfully stimulates gonadotrophin secretion in the ewe: evidence for a seasonal threshold of sensitivity". Journal of Neuroendocrinology. 24 (5): 725–36. doi:10.1111/j.1365-2826.2012.02283.x. PMID 22283564. S2CID 25770293.
- ↑ Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K (March 2006). "Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail". Endocrinology. 147 (3): 1187–94. doi: 10.1210/en.2005-1178 . PMID 16293662.
- ↑ Tsutsui K, Bentley GE, Ubuka T, Saigoh E, Yin H, Osugi T, Inoue K, Chowdhury VS, Ukena K, Ciccone N, Sharp PJ, Wingfield JC (2007-08-01). "The general and comparative biology of gonadotropin-inhibitory hormone (GnIH)". General and Comparative Endocrinology. Proceedings of the 23rd Conference of European Comparative Endocrinologists: Part 2. 153 (1–3): 365–70. doi:10.1016/j.ygcen.2006.10.005. PMID 17141777.
- ↑ Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC (April 2006). "Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH)". Hormones and Behavior. 49 (4): 550–5. doi:10.1016/j.yhbeh.2005.12.005. PMID 16460739. S2CID 1801090.
- ↑ Paullada-Salmerón JA, Cowan M, Aliaga-Guerrero M, Morano F, Zanuy S, Muñoz-Cueto JA (June 2016). "Gonadotropin Inhibitory Hormone Down-Regulates the Brain-Pituitary Reproductive Axis of Male European Sea Bass (Dicentrarchus labrax)". Biology of Reproduction. 94 (6): 121. doi:10.1095/biolreprod.116.139022. PMC 6322450 . PMID 26984999.
- ↑ Tsutsui K, Ubuka T (2016). "GnIH Control of Feeding and Reproductive Behaviors". Frontiers in Endocrinology. 7: 170. doi: 10.3389/fendo.2016.00170 . PMC 5186799 . PMID 28082949.
- ↑ Ubuka T, Ukena K, Sharp P, Bentley G, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006; 147, 1187-1194. doi: 10.1210/en.2005-1178
- 1 2 Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ (July 2012). "Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect". General and Comparative Endocrinology. Profiles in Comparative Endocrinology: Eric Roubos. 177 (3): 305–14. doi:10.1016/j.ygcen.2012.02.013. PMC 3378827 . PMID 22391238.
- ↑ Singh P, Krishna A, Tsutsui K (March 2011). "Effects of gonadotropin-inhibitory hormone on folliculogenesis and steroidogenesis of cyclic mice". Fertility and Sterility. 95 (4): 1397–404. doi:10.1016/j.fertnstert.2010.03.052. PMID 20452585.
- ↑ Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D (July 2009). "Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats". Proceedings of the National Academy of Sciences of the United States of America. 106 (27): 11324–9. Bibcode:2009PNAS..10611324K. doi: 10.1073/pnas.0901176106 . PMC 2698887 . PMID 19541621.
- ↑ Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ (July 2010). "Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function". Frontiers in Neuroendocrinology. 31 (3): 284–95. doi:10.1016/j.yfrne.2010.03.001. PMID 20211640. S2CID 10120758.
- ↑ Kiyohara M, Son YL, Tsutsui K (April 2017). "Involvement of gonadotropin-inhibitory hormone in pubertal disorders induced by thyroid status". Scientific Reports. 7 (1): 1042. Bibcode:2017NatSR...7.1042K. doi:10.1038/s41598-017-01183-8. PMC 5430760 . PMID 28432332.
- ↑ Shinomiya A, Shimmura T, Nishiwaki-Ohkawa T, Yoshimura T (2014). "Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone". Frontiers in Endocrinology. 5: 12. doi: 10.3389/fendo.2014.00012 . PMC 3930870 . PMID 24600435.