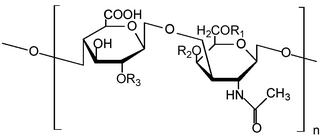
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. In this form, HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.
In enzymology, a [heparan sulfate]-glucosamine 3-sulfotransferase 1 is an enzyme that catalyzes the chemical reaction
In enzymology, a [heparan sulfate]-glucosamine 3-sulfotransferase 2 is an enzyme that catalyzes the chemical reaction
In enzymology, a [heparan sulfate]-glucosamine 3-sulfotransferase 3 is an enzyme that catalyzes the chemical reaction

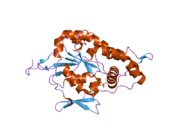
Bifunctional heparan sulfate N-deacetylase/N-sulfotransferase 1 is an enzyme. In humans, it is encoded by the NDST1 gene.

Carbohydrate sulfotransferase 5 is an enzyme that in humans is encoded by the CHST5 gene.

Heparan sulfate glucosamine 3-O-sulfotransferase 3A1 is an enzyme that in humans is encoded by the HS3ST3A1 gene.

Bifunctional heparan sulfate N-deacetylase/N-sulfotransferase 2 is an enzyme that in humans is encoded by the NDST2 gene.

Heparan sulfate 2-O-sulfotransferase 1 is an enzyme that in humans is encoded by the HS2ST1 gene.

Heparan sulfate glucosamine 3-O-sulfotransferase 3B1 is an enzyme that in humans is encoded by the HS3ST3B1 gene. Heparan sulfate biosynthetic enzymes are key components in generating myriad distinct heparan sulfate fine structures that carry out multiple biologic activities. The enzyme encoded by this gene is a member of the heparan sulfate biosynthetic enzyme family. It is a type II integral membrane protein and possesses heparan sulfate glucosaminyl 3-O-sulfotransferase activity ( HS3ST3A1). The Sulfotransferase domain of this enzyme is highly similar to the same domain of heparan sulfate D-glucosaminyl 3-O-sulfotransferase 3A1 and these two enzymes sulfate an identical disaccharide. This gene is widely expressed, with the most abundant expression in liver and placenta.

Carbohydrate sulfotransferase 7 is an enzyme that in humans is encoded by the CHST7 gene.

Bifunctional heparan sulfate N-deacetylase/N-sulfotransferase 3 is an enzyme that in humans is encoded by the NDST3 gene. It catalyses the reaction:
3'-phosphoadenylyl sulfate + α-D-glucosaminyl-[heparan sulfate](n) = adenosine 3',5'-bisphosphate + 2 H+ + N-sulfo-α-D-glucosaminyl-[heparan sulfate](n)

Heparan sulfate glucosamine 3-O-sulfotransferase 2 is an enzyme that in humans is encoded by the HS3ST2 gene.

In biochemistry, carbohydrate sulfotransferases are enzymes within the class of sulfotransferases which catalyze the transfer of the sulfate functional group to carbohydrate groups in glycoproteins and glycolipids. Carbohydrates are used by cells for a wide range of functions from structural purposes to extracellular communication. Carbohydrates are suitable for such a wide variety of functions due to the diversity in structure generated from monosaccharide composition, glycosidic linkage positions, chain branching, and covalent modification. Possible covalent modifications include acetylation, methylation, phosphorylation, and sulfation. Sulfation, performed by carbohydrate sulfotransferases, generates carbohydrate sulfate esters. These sulfate esters are only located extracellularly, whether through excretion into the extracellular matrix (ECM) or by presentation on the cell surface. As extracellular compounds, sulfated carbohydrates are mediators of intercellular communication, cellular adhesion, and ECM maintenance.
Glucuronyl-galactosyl-proteoglycan 4-alpha-N-acetylglucosaminyltransferase is an enzyme with systematic name UDP-N-acetyl-D-glucosamine:beta-D-glucuronosyl-(1->3)-beta-D-galactosyl-(1->3)-beta-D-galactosyl-(1->4)-beta-D-xylosyl-proteoglycan 4IV-alpha-N-acetyl-D-glucosaminyltransferase. This enzyme catalyses the following chemical reaction
Glucuronosyl-N-acetylglucosaminyl-proteoglycan 4-alpha-N-acetylglucosaminyltransferase is an enzyme with systematic name UDP-N-acetyl-D-glucosamine:beta-D-glucuronosyl-(1->4)-N-acetyl-alpha-D-glucosaminyl-proteoglycan 4-alpha-N-acetylglucosaminyltransferase. This enzyme catalyses the following chemical reaction
Glucosaminyl 3-O-sulfotransferase may refer to:
Heparanase is an enzyme with systematic name heparan sulfate N-sulfo-D-glucosamine endoglucanase. This enzyme catalyses the following chemical reaction
Jian Liu is a John & Deborah S. McNeill, Jr. Distinguished Professor at the UNC Eshelman School of Pharmacy, at the University of North Carolina at Chapel Hill. He is also a founder and the chief scientific officer at Glycan Therapeutics.