Related Research Articles
Nanomedicine is the medical application of nanotechnology. Nanomedicine ranges from the medical applications of nanomaterials and biological devices, to nanoelectronic biosensors, and even possible future applications of molecular nanotechnology such as biological machines. Current problems for nanomedicine involve understanding the issues related to toxicity and environmental impact of nanoscale materials.
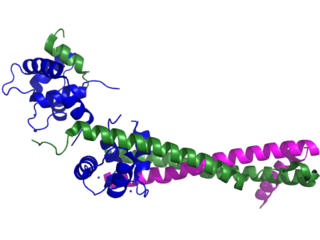
Troponin, or the troponin complex, is a complex of three regulatory proteins that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocarditis, myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low.

The endocardium is the innermost layer of tissue that lines the chambers of the heart. Its cells are embryologically and biologically similar to the endothelial cells that line blood vessels. The endocardium also provides protection to the valves and heart chambers.
Stem-cell therapy uses stem cells to treat or prevent a disease or condition. As of 2024, the only FDA-approved therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone marrow or peripheral blood stem cell transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases and conditions such as diabetes and heart disease.
Targeted drug delivery, sometimes called smart drug delivery, is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult, and the reduced ability to adjust the dosages.
Cardiomyoplasty is a surgical procedure in which healthy muscle from another part of the body is wrapped around the heart to provide support for the failing heart. Most often the latissimus dorsi muscle is used for this purpose. A special pacemaker is implanted to make the skeletal muscle contract. If cardiomyoplasty is successful and increased cardiac output is achieved, it usually acts as a bridging therapy, giving time for damaged myocardium to be treated in other ways, such as remodeling by cellular therapies.

A coronary stent is a tube-shaped device placed in the coronary arteries that supply blood to the heart, to keep the arteries open in patients suffering from coronary heart disease. The vast majority of stents used in modern interventional cardiology are drug-eluting stents (DES). They are used in a medical procedure called percutaneous coronary intervention (PCI). Coronary stents are divided into two broad types: drug-eluting and bare metal stents. As of 2023, drug-eluting stents were used in more than 90% of all PCI procedures. Stents reduce angina and have been shown to improve survival and decrease adverse events after a patient has suffered a heart attack—medically termed an acute myocardial infarction.

A biointerface is the region of contact between a biomolecule, cell, biological tissue or living organism or organic material considered living with another biomaterial or inorganic/organic material. The motivation for biointerface science stems from the urgent need to increase the understanding of interactions between biomolecules and surfaces. The behavior of complex macromolecular systems at materials interfaces are important in the fields of biology, biotechnology, diagnostics, and medicine. Biointerface science is a multidisciplinary field in which biochemists who synthesize novel classes of biomolecules cooperate with scientists who have developed the tools to position biomolecules with molecular precision, scientists who have developed new spectroscopic techniques to interrogate these molecules at the solid-liquid interface, and people who integrate these into functional devices. Well-designed biointerfaces would facilitate desirable interactions by providing optimized surfaces where biological matter can interact with other inorganic or organic materials, such as by promoting cell and tissue adhesion onto a surface.

A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops in one of the coronary arteries of the heart, causing infarction to the heart muscle. The most common symptom is retrosternal chest pain or discomfort that classically radiates to the left shoulder, arm, or jaw. The pain may occasionally feel like heartburn. This is the dangerous type of Acute coronary syndrome.
Nano-scaffolding or nanoscaffolding is a medical process used to regrow tissue and bone, including limbs and organs. The nano-scaffold is a three-dimensional structure composed of polymer fibers very small that are scaled from a Nanometer scale. Developed by the American military, the medical technology uses a microscopic apparatus made of fine polymer fibers called a scaffold. Damaged cells grip to the scaffold and begin to rebuild missing bone and tissue through tiny holes in the scaffold. As tissue grows, the scaffold is absorbed into the body and disappears completely.
Amniotic stem cells are the mixture of stem cells that can be obtained from the amniotic fluid as well as the amniotic membrane. They can develop into various tissue types including skin, cartilage, cardiac tissue, nerves, muscle, and bone. The cells also have potential medical applications, especially in organ regeneration.

Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from Drosophila to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins. The discovery of RGD and elucidation of how RGD binds to integrins has led to the development of a number of drugs and diagnostics, while the peptide itself is used ubiquitously in bioengineering. Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion.

Myocardial infarction complications may occur immediately following a myocardial infarction, or may need time to develop. After an infarction, an obvious complication is a second infarction, which may occur in the domain of another atherosclerotic coronary artery, or in the same zone if there are any live cells left in the infarct.
Human HGF plasmid DNA therapy of cardiomyocytes is being examined as a potential treatment for coronary artery disease, as well as treatment for the damage that occurs to the heart after MI. After MI, the myocardium suffers from reperfusion injury which leads to death of cardiomyocytes and detrimental remodelling of the heart, consequently reducing proper cardiac function. Transfection of cardiac myocytes with human HGF reduces ischemic reperfusion injury after MI. The benefits of HGF therapy include preventing improper remodelling of the heart and ameliorating heart dysfunction post-MI.
Human engineered cardiac tissues (hECTs) are derived by experimental manipulation of pluripotent stem cells, such as human embryonic stem cells (hESCs) and, more recently, human induced pluripotent stem cells (hiPSCs) to differentiate into human cardiomyocytes. Interest in these bioengineered cardiac tissues has risen due to their potential use in cardiovascular research and clinical therapies. These tissues provide a unique in vitro model to study cardiac physiology with a species-specific advantage over cultured animal cells in experimental studies. hECTs also have therapeutic potential for in vivo regeneration of heart muscle. hECTs provide a valuable resource to reproduce the normal development of human heart tissue, understand the development of human cardiovascular disease (CVD), and may lead to engineered tissue-based therapies for CVD patients.
Regeneration in humans is the regrowth of lost tissues or organs in response to injury. This is in contrast to wound healing, or partial regeneration, which involves closing up the injury site with some gradation of scar tissue. Some tissues such as skin, the vas deferens, and large organs including the liver can regrow quite readily, while others have been thought to have little or no capacity for regeneration following an injury.
Eosinophilic myocarditis is inflammation in the heart muscle that is caused by the infiltration and destructive activity of a type of white blood cell, the eosinophil. Typically, the disorder is associated with hypereosinophilia, i.e. an eosinophil blood cell count greater than 1,500 per microliter. It is distinguished from non-eosinophilic myocarditis, which is heart inflammation caused by other types of white blood cells, i.e. lymphocytes and monocytes, as well as the respective descendants of these cells, NK cells and macrophages. This distinction is important because the eosinophil-based disorder is due to a particular set of underlying diseases and its preferred treatments differ from those for non-eosinophilic myocarditis.
Tissue engineered heart valves (TEHV) offer a new and advancing proposed treatment of creating a living heart valve for people who are in need of either a full or partial heart valve replacement. Currently, there are over a quarter of a million prosthetic heart valves implanted annually, and the number of patients requiring replacement surgeries is only suspected to rise and even triple over the next fifty years. While current treatments offered such as mechanical valves or biological valves are not deleterious to one's health, they both have their own limitations in that mechanical valves necessitate the lifelong use of anticoagulants while biological valves are susceptible to structural degradation and reoperation. Thus, in situ (in its original position or place) tissue engineering of heart valves serves as a novel approach that explores the use creating a living heart valve composed of the host's own cells that is capable of growing, adapting, and interacting within the human body's biological system.

Erin Baker Lavik is an American bioengineer serving as the deputy director and chief technology officer of the National Cancer Institute's Division of Cancer Prevention (DCP) since 2023. She was previously a professor of chemical, biochemical, and environmental engineering at the University of Maryland, Baltimore County. Lavik develops polymers and nanoparticles that can protect the nervous system. She is a fellow of the American Institute for Medical and Biological Engineering.

Milica Radisic is a Serbian Canadian tissue engineer, academic and researcher. She is a professor at the University of Toronto’s Institute of Biomaterials and Biomedical Engineering, and the Department of Chemical Engineering and Applied Chemistry. She co-founded TARA Biosystems and is a senior scientist at the Toronto General Hospital Research Institute.
References
- ↑ Mahmoudi M, Yu M, Serpooshan V, Wu JC, Langer R, Lee RT, Karp JM, Farokhzad OC (2017). "Multiscale technologies for treatment of ischemic cardiomyopathy". Nature Nanotechnology. 12 (9): 845–855. doi:10.1038/nnano.2017.167. PMC 5717755 . PMID 28875984.
- 1 2 3 4 5 6 7 8 9 10 11 12 Fliesler, Nancy (30 September 2011). "Could Nanotechnology Improve Treatment of Heart Attack and Heart Failure?". Mass Device. Retrieved 2019-04-11.
- 1 2 3 4 Heffernan NJ, Murthy N (2005). "Polyketal nanoparticles: A new pH-sensitive biodegradable drug delivery vehicle". Bioconjugate Chemistry. 16 (6): 1340–1342. doi:10.1021/bc050176w. PMID 16287226.
- ↑ Fu K, Pack DW, Klibanov AM, Langer R (2000). "Visual evidence of acidic environment within degrading poly-(lactic-co-glycolic acid) (PLGA) microspheres". Pharm. Res. 17 (1): 100–106. doi:10.1023/A:1007582911958. PMID 10714616. S2CID 22378621.
- 1 2 Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM (2008). "Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction". Hypertension. 51 (2): 319–325. CiteSeerX 10.1.1.539.7577 . doi: 10.1161/hypertensionaha.107.101980 . PMID 18180403.
- ↑ Krijnen PA, Meischl C, Hack CE, Meijer CJ, Visser CA, Roos D, Niessen HW (2003). "Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction". Journal of Clinical Pathology. 56 (3): 194–199. doi: 10.1136/jcp.56.3.194 . PMC 1769897 . PMID 12610097.
- 1 2 3 Somasuntharam I, Boopathy AV, Khan RS, Martinez MD, Brown ME, Murthy N, Davis ME (2013). "Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction". Biomaterials. 34 (31): 7790–7798. doi:10.1016/j.biomaterials.2013.06.051. PMC 3766948 . PMID 23856052.
- 1 2 Sheshadri G, Sy JC, Brown M, Dikalov S, Yang SC, Murthy N, Davis ME (2010). "The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury". Biomaterials. 31 (6): 1372–1379. doi:10.1016/j.biomaterials.2009.10.045. PMC 2813932 . PMID 19889454.
- ↑ Khaper N, Kaur K, Li T, Farahmand F, Singal PK (2003). "Antioxidant enzyme gene expression in congestive heart failure following myocardial infarction". Molecular and Cellular Biochemistry. 251 (1–2): 9–15. doi:10.1023/A:1025448908694. PMID 14575298. S2CID 2610363.
- 1 2 3 Sy JC, Sheshadri G, Yang SC, Brown M, Oh T, Dikalov S, Murthy N, Davis ME (2008). "Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction". Nature. 7 (11): 863–869. doi:10.1038/nmat2299. PMC 2705946 . PMID 18931671.