Related Research Articles
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of the N-oxide to form an alkene and a hydroxylamine.
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes.
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound. A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.
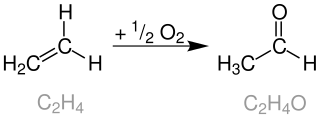
The Wacker process or the Hoechst-Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride and copper(II) chloride as the catalyst. This chemical reaction was one of the first homogeneous catalysis with organopalladium chemistry applied on an industrial scale.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and a dienophile are both part of the same molecule. The reaction leads to the formation of the same cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system. This reaction gives rise to various natural derivatives of decalin.

Richard Frederick Heck was an American chemist noted for the discovery and development of the Heck reaction, which uses palladium to catalyze organic chemical reactions that couple aryl halides with alkenes. The analgesic naproxen is an example of a compound that is prepared industrially using the Heck reaction.

The Liebeskind–Srogl coupling reaction is an organic reaction forming a new carbon–carbon bond from a thioester and a boronic acid using a metal catalyst. It is a cross-coupling reaction. This reaction was invented by and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory University, Atlanta, Georgia, USA. There are three generations of this reaction, with the first generation shown below. The original transformation used catalytic Pd(0), TFP = tris(2-furyl)phosphine as an additional ligand and stoichiometric CuTC = copper(I) thiophene-2-carboxylate as a co-metal catalyst. The overall reaction scheme is shown below.
Electrophilic amination is a chemical process involving the formation of a carbon–nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen.
M. Christina White is a professor of chemistry at the University of Illinois at Urbana-Champaign. Her research in the field of organometallic catalysis focuses on developing highly selective carbon–hydrogen bond activation methods to streamline the process of complex molecule synthesis.
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.

The Catellani reaction was discovered by Marta Catellani and co-workers in 1997. The reaction uses aryl iodides to perform bi- or tri-functionalization, including C-H functionalization of the unsubstituted ortho position(s), followed a terminating cross-coupling reaction at the ipso position. This cross-coupling cascade reaction depends on the ortho-directing transient mediator, norbornene.
In organic chemistry, the Fujiwara–Moritani reaction is a type of cross coupling reaction where an aromatic C-H bond is directly coupled to an olefinic C-H bond, generating a new C-C bond. This reaction is performed in the presence of a transition metal, typically palladium. The reaction was discovered by Yuzo Fujiwara and Ichiro Moritani in 1967. An external oxidant is required to this reaction to be run catalytically. Thus, this reaction can be classified as a C-H activation reaction, an oxidative Heck reaction, and a C-H olefination. Surprisingly, the Fujiwara–Moritani reaction was discovered before the Heck reaction.
Dialkylbiaryl phosphine ligands are phosphine ligands that are used in homogeneous catalysis. They have proved useful in Buchwald-Hartwig amination and etherification reactions as well as Negishi cross-coupling, Suzuki-Miyaura cross-coupling, and related reactions. In addition to these Pd-based processes, their use has also been extended to transformations catalyzed by nickel, gold, silver, copper, rhodium, and ruthenium, among other transition metals.

The Mizoroki−Heck coupling of aryl halides and alkenes to form C(sp2)–C(sp2) bonds has become a staple transformation in organic synthesis, owing to its broad functional group compatibility and varied scope. In stark contrast, the palladium-catalyzed reductive Heck reaction has received considerably less attention, despite the fact that early reports of this reaction date back almost half a century. From the perspective of retrosynthetic logic, this transformation is highly enabling because it can forge alkyl–aryl linkages from widely available alkenes, rather than from the less accessible and/or more expensive alkyl halide or organometallic C(sp3) synthons that are needed in a classical aryl/alkyl cross-coupling.
Carbonyl olefin metathesis is a type of metathesis reaction that entails, formally, the redistribution of fragments of an alkene and a carbonyl by the scission and regeneration of carbon-carbon and carbon-oxygen double bonds respectively. It is a powerful method in organic synthesis using simple carbonyls and olefins and converting them into less accessible products with higher structural complexity.
F. Dean Toste is the Gerald E. K. Branch Distinguished Professor of Chemistry at the University of California, Berkeley and faculty scientist at the chemical sciences division of Lawrence Berkeley National Lab. He is a prominent figure in the field of organic chemistry and is best known for his contributions to gold chemistry and asymmetric ion-pairing catalysis. Toste was elected a member of the National Academy of Sciences in 2020, and a member of the American Academy of Arts and Sciences in 2018.
The Cadogan–Sundberg indole synthesis, or simply Cadogan indole synthesis, is a name reaction in organic chemistry that allows for the generation of indoles from o-nitrostyrenes with the use of trialkyl phosphites, such as triethyl phosphite.
References
- 1 2 Hegedus, Louis S.; Allen, Gary F.; Waterman, Edward L. (1976-04-01). "Palladium assisted intramolecular amination of olefins. A new synthesis of indoles". Journal of the American Chemical Society. 98 (9): 2674–2676. doi:10.1021/ja00425a051.
- ↑ Hegedus, Louis S.; Allen, Gary F.; Bozell, Joseph J.; Waterman, Edward L. (1978-08-01). "Palladium-assisted intramolecular amination of olefins. Synthesis of nitrogen heterocycles". Journal of the American Chemical Society. 100 (18): 5800–5807. doi:10.1021/ja00486a035.
- ↑ Hegedus, Louis S.; Winton, Peter M.; Varaprath, Sudarsanan (1981-05-01). "Palladium-assisted N-alkylation of indoles: attempted application to polycyclization". The Journal of Organic Chemistry. 46 (11): 2215–2221. doi:10.1021/jo00324a004.
- 1 2 Harrington, Peter J.; Hegedus, Louis S. (1984-07-01). "Palladium-catalyzed reactions in the synthesis of 3- and 4-substituted indoles. Approaches to ergot alkaloids". The Journal of Organic Chemistry. 49 (15): 2657–2662. doi:10.1021/jo00189a001.
- ↑ Wang, Zerong (15 September 2010). Comprehensive Organic Name Reactions and Reagents. doi:10.1002/9780470638859.conrr302. ISBN 9780470638859.
- ↑ Li, Jie Jack (2006). Name Reactions A Collection of Detailed Reaction Mechanisms. Springer. p. 289. ISBN 978-3-540-30030-4.