Related Research Articles

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the very first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.

CD34 is a transmembrane phosphoglycoprotein protein encoded by the CD34 gene in humans, mice, rats and other species.

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.

Extramedullary hematopoiesis refers to hematopoiesis occurring outside of the medulla of the bone. It can be physiologic or pathologic.
The aorta-gonad-mesonephros (AGM) is a region of embryonic mesoderm that develops during embryonic development from the para-aortic splanchnopleura in chick, mouse and human embryos. The very first adult definitive haematopoietic stem cells, capable of long-term multilineage repopulation of adult irradiated recipients, originate from the ventral endothelial wall of the embryonic dorsal aorta, through an endothelial transdifferentiation process referred to as an 'endothelial-to-haematopoietic transition' (EHT). In the mouse embryo, these very first HSCs are characterised by their expression of Ly6A-GFP (Sca1), CD31, CD34, cKit, CD27, CD41, Gata2, Runx1, Notch1, and BMP amongst others.
Angioblasts are embryonic cells from which the endothelium of blood vessels arises. They are derived from embryonic mesoderm. Blood vessels first make their appearance in several scattered vascular areas that are developed simultaneously between the endoderm and the mesoderm of the yolk-sac, i. e., outside the body of the embryo. Here a new type of cell, the angioblast, is differentiated from the mesoderm.
Endothelial stem cells (ESCs) are one of three types of stem cells found in bone marrow. They are multipotent, which describes the ability to give rise to many cell types, whereas a pluripotent stem cell can give rise to all types. ESCs have the characteristic properties of a stem cell: self-renewal and differentiation. These parent stem cells, ESCs, give rise to progenitor cells, which are intermediate stem cells that lose potency. Progenitor stem cells are committed to differentiating along a particular cell developmental pathway. ESCs will eventually produce endothelial cells (ECs), which create the thin-walled endothelium that lines the inner surface of blood vessels and lymphatic vessels. The lymphatic vessels include things such as arteries and veins. Endothelial cells can be found throughout the whole vascular system and they also play a vital role in the movement of white blood cells

A mesoangioblast is a type of progenitor cell that is associated with vasculature walls. Mesoangioblasts exhibit many similarities to pericytes, which are found in the small vessels. Mesoangioblasts are multipotent stem cells with the potential to progress down the endothelial or mesodermal lineages. Mesoangioblasts express the critical marker of angiopoietic progenitors, KDR (FLK1). Because of these properties, mesoangioblasts are a precursor of skeletal, smooth, and cardiac muscle cells along with endothelial cells. Research has suggested their application for stem cell therapies for muscular dystrophy and cardiovascular disease.
Endothelial progenitor cell is a term that has been applied to multiple different cell types that play roles in the regeneration of the endothelial lining of blood vessels. Outgrowth endothelial cells are an EPC subtype committed to endothelial cell formation. Despite the history and controversy, the EPC in all its forms remains a promising target of regenerative medicine research.
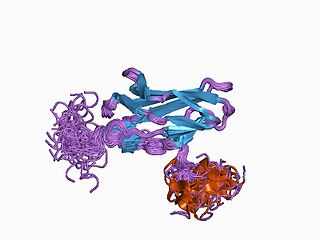
Runt-related transcription factor 1 (RUNX1) also known as acute myeloid leukemia 1 protein (AML1) or core-binding factor subunit alpha-2 (CBFA2) is a protein that in humans is encoded by the RUNX1 gene.

Homeobox protein Hox-A9 is a protein that in humans is encoded by the HOXA9 gene.

In cell biology, precursor cells—also called blast cells—are partially differentiated, or intermediate, and are sometimes referred to as progenitor cells. A precursor cell is a stem cell with the capacity to differentiate into only one cell type, meaning they are unipotent stem cells. In embryology, precursor cells are a group of cells that later differentiate into one organ. However, progenitor cells are considered multipotent.
Stem cell markers are genes and their protein products used by scientists to isolate and identify stem cells. Stem cells can also be identified by functional assays. Below is a list of genes/protein products that can be used to identify various types of stem cells, or functional assays that do the same. The initial version of the list below was obtained by mining the PubMed database as described in

Megakaryocyte–erythroid progenitor cells, among other blood cells, are generated as a result of hematopoiesis, which occurs in the bone marrow. Hematopoietic stem cells can differentiate into one of two progenitor cells: the common lymphoid progenitor and the common myeloid progenitor. MEPs derive from the common myeloid progenitor lineage. Megakaryocyte/erythrocyte progenitor cells must commit to becoming either platelet-producing megakaryocytes via megakaryopoiesis or erythrocyte-producing erythroblasts via erythropoiesis. Most of the blood cells produced in the bone marrow during hematopoiesis come from megakaryocyte/erythrocyte progenitor cells.

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.
Many human blood cells, such as red blood cells (RBCs), immune cells, and even platelets all originate from the same progenitor cell, the hematopoietic stem cell (HSC). As these cells are short-lived, there needs to be a steady turnover of new blood cells and the maintenance of an HSC pool. This is broadly termed hematopoiesis. This event requires a special environment, termed the hematopoietic stem cell niche, which provides the protection and signals necessary to carry out the differentiation of cells from HSC progenitors. This niche relocates from the yolk sac to eventually rest in the bone marrow of mammals. Many pathological states can arise from disturbances in this niche environment, highlighting its importance in maintaining hematopoiesis.
Gordon M. Keller is a Canadian scientist recognized for his research on applying developmental biology findings to in vitro pluripotent stem cell differentiation. He is currently a Senior Scientist at the Ontario Cancer Institute, a Professor at the University of Toronto and the director of the McEwen Centre for Regenerative Medicine.

Musashi-2, also known as Musashi RNA binding protein 2, is a protein that in humans is encoded by the MSI2 gene. Like its homologue musashi-1 (MSI1), it is an RNA-binding protein involved in stemness.

Islet resident macrophages are the predominant myeloid cell of the pancreatic islets of langerhans.
OP9 cells are a cell line derived from mouse bone marrow stromal cells (mesenchyme). These cells are now characterized as stem cells. When co-cultured with embryonic stem cells (ESC), OP9 cells can induce ESC to differentiate into blood cells by serving as a feeder layer. They have the potential to be used in cell therapy, regenerative medicine and as immunomodulators.
References
- ↑ Basak GW, Yasukawa S, Alfaro A, et al. (2009). "Human embryonic stem cells hemangioblast express HLA-antigens". J Transl Med. 7 (1): 27. doi: 10.1186/1479-5876-7-27 . PMC 2680830 . PMID 19386101.
- ↑ Miki Takeuchi; Yuji Fusei; Mana Watanabe; Christina-Sylvia Andrea; Miho Takeuchi; Hitomi Nakajima; Ken Ohashi; Hiroshi Kaneko; Maki Kobayashi-Osak; Masayuki Yamamoto; Makoto Kobayashia (2015). "LSD1/KDM1A promotes hematopoietic commitment of hemangioblasts through downregulation of Etv2". Proceedings of the National Academy of Sciences of the United States of America. 112 (45): 13922–13927. Bibcode:2015PNAS..11213922T. doi: 10.1073/pnas.1517326112 . PMC 4653156 . PMID 26512114.
- ↑ Hemangioblasts at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Vogeli KM, Jin SW, Martin GR, Stainier DY (September 2006). "A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula". Nature. 443 (7109): 337–9. Bibcode:2006Natur.443..337V. doi:10.1038/nature05045. PMID 16988712. S2CID 4300264.
- ↑ Loges S, et al. (2004). "Identification of the Adult Hemangioblast". Stem Cells and Development. 13 (1): 229–42. doi: 10.1089/154732804323099163 . PMID 15186719.
- ↑ Sabin F (2002). "Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick (1917)". J Hematother Stem Cell Res. 11 (1): 5–7. doi:10.1089/152581602753448496. PMID 11846999.
- ↑ Murray PDF (1932). "The development in vitro of the blood of early chick embryo". Proceedings of the Royal Society. 111 (773): 497–521. Bibcode:1932RSPSB.111..497M. doi: 10.1098/rspb.1932.0070 .
- ↑ Zambidis ET, Park TS, Yu W, et al. (2008). "Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells". Blood. 112 (9): 3601–14. doi:10.1182/blood-2008-03-144766. PMC 2572789 . PMID 18728246.
- ↑ Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller GA (1997). "A common precursor for primitive erythropoisis and definitive hematopoiesis". Nature. 386 (6624): 488–93. Bibcode:1997Natur.386..488K. doi:10.1038/386488a0. PMID 9087406. S2CID 4350178.
- ↑ Choi K, Kennedy M, Kazarov A, et al. (1998). "A common precursor for hematopoietic and endothelial cells". Development. 125 (4): 725–32. doi:10.1242/dev.125.4.725. PMID 9435292.
- ↑ Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G (2004). "Haemangioblast commitment is initiated in the primitive streak of the mouse embryo". Nature. 432 (7017): 625–30. Bibcode:2004Natur.432..625H. doi:10.1038/nature03122. PMID 15577911. S2CID 4347714.