Related Research Articles

Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.

A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

An HIV vaccine is a potential vaccine that could be either a preventive vaccine or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV or treat HIV-infected individuals.
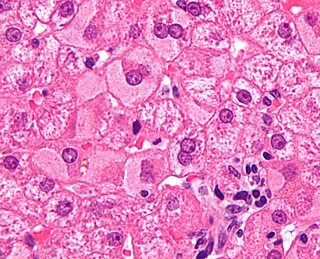
Viral hepatitis is liver inflammation due to a viral infection. It may present in acute form as a recent infection with relatively rapid onset, or in chronic form.

The hepatitis C virus (HCV) is a small, enveloped, positive-sense single-stranded RNA virus of the family Flaviviridae. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer and lymphomas in humans.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. Both in-vivo assembly and in-vitro assembly have been successfully shown to form virus-like particles. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.
HLA class II histocompatibility antigen gamma chain also known as HLA-DR antigens-associated invariant chain or CD74, is a protein that in humans is encoded by the CD74 gene. The invariant chain is a polypeptide which plays a critical role in antigen presentation. It is involved in the formation and transport of MHC class II peptide complexes for the generation of CD4+ T cell responses. The cell surface form of the invariant chain is known as CD74. CD74 is a cell surface receptor for the cytokine macrophage migration inhibitory factor (MIF).

Hepatitis B is an infectious disease caused by the Hepatitis B virus (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection.
Immune stimulating complexes (ISCOMs) are spherical open cage-like structures (typically 40 nm in diameter) that are spontaneously formed when mixing together cholesterol, phospholipids and Quillaja saponins under a specific stoichiometry. The complex displays immune stimulating properties and is thus mainly used as a vaccine adjuvant in order to induce a stronger immune response and longer protection.
A subunit vaccine is a vaccine that contains purified parts of the pathogen that are antigenic, or necessary to elicit a protective immune response. Subunit vaccine can be made from dissembled viral particles in cell culture or recombinant DNA expression, in which case it is a recombinant subunit vaccine.
Peptide-based synthetic vaccines, also called epitope vaccines, are subunit vaccines made from peptides. The peptides mimic the epitopes of the antigen that triggers direct or potent immune responses. Peptide vaccines can not only induce protection against infectious pathogens and non-infectious diseases but also be utilized as therapeutic cancer vaccines, where peptides from tumor-associated antigens are used to induce an effective anti-tumor T-cell response.

Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV). It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication. Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK. The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4's (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.
Intrastructural help (ISH) is where T and B cells cooperate to help or suppress an immune response gene. ISH has proven effective for the treatment of influenza, rabies related lyssavirus, hepatitis B, and the HIV virus. This process was used in 1979 to observe that T cells specific to the influenza virus could promote the stimulation of hemagglutinin specific B cells and elicit an effective humoral immune response. It was later applied to the lyssavirus and was shown to protect raccoons from lethal challenge. The ISH principle is especially beneficial because relatively invariable structural antigens can be used for the priming of T-cells to induce humoral immune response against variable surface antigens. Thus, the approach has also transferred well for the treatment of hepatitis B and HIV.

Interferon lambda 4 is one of the most recently discovered human genes and the newest addition to the interferon lambda protein family. This gene encodes the IFNL4 protein, which is involved in immune response to viral infection.

CoVLP was a COVID-19 vaccine developed by Medicago in Canada and GlaxoSmithKline (GSK). The product and Medicago, Inc. were owned by Mitsubishi who terminated the company and program in February 2023 due to high international market competition for COVID-19 vaccines.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.

Riccardo Cortese was an Italian scientist, entrepreneur, and innovator in the field of gene expression, drug discovery and genetic vaccines. His work led to the development of novel therapeutic strategies for the prevention and cure of viral infections, including HIV, HCV, Ebola and RSV. He pioneered a novel platform technology based on simian adenoviral vectors for prophylactic and therapeutic vaccines, and authored more than 300 publications in peer reviewed journals in the field of gene expression, transcriptional control, molecular virology and immunology.
References
- ↑ Randal J (June 1999). "Hepatitis C vaccine hampered by viral complexity, many technical restraints". Journal of the National Cancer Institute. 91 (11): 906–8. doi:10.1093/jnci/91.11.906. PMID 10359539.
- ↑ Strickland GT, El-Kamary SS, Klenerman P, Nicosia A (June 2008). "Hepatitis C vaccine: supply and demand". The Lancet. Infectious Diseases. 8 (6): 379–86. doi:10.1016/S1473-3099(08)70126-9. PMID 18501853.
- ↑ "Hepatitis C Questions and Answers for the Public". Centers for Disease Control and Prevention . 10 September 2019. Retrieved 20 April 2023.
- ↑ "Scripps Research Institute Scientists Achieve Most Detailed Picture Ever of Key Part of Hepatitis C Virus" (Press release). Scripps Research Institute. 28 November 2013. Retrieved 6 December 2013.
- ↑ "The hepatitis C virus". WHO. Archived from the original on 4 October 2013. Retrieved 1 October 2013.
- ↑ Torresi J (7 November 2017). "The Rationale for a Preventative HCV Virus-Like Particle (VLP) Vaccine". Frontiers in Microbiology. 8: 2163. doi: 10.3389/fmicb.2017.02163 . PMC 5674006 . PMID 29163442.
- 1 2 Lombardi A, Mondelli MU (March 2019). "Hepatitis C: Is eradication possible?". Liver International. 39 (3): 416–426. doi: 10.1111/liv.14011 . PMID 30472772.
- ↑ Latimer B, Toporovski R, Yan J, Pankhong P, Morrow MP, Khan AS, et al. (20 June 2014). "Strong HCV NS3/4a, NS4b, NS5a, NS5b-specific cellular immune responses induced in Rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine". Human Vaccines & Immunotherapeutics. 10 (8): 2357–65. doi:10.4161/hv.29590. PMC 4896772 . PMID 25424943.
- ↑ Clinical trial number NCT02772003 for "DNA Vaccine Therapy in Treating Patients With Chronic Hepatitis C Virus Infection" at ClinicalTrials.gov
- ↑ Esposito I, Cicconi P, D'Alise AM, Brown A, Esposito M, Swadling L, et al. (June 2020). "MHC class II invariant chain-adjuvanted viral vectored vaccines enhances T cell responses in humans" (PDF). Science Translational Medicine. 12 (548): eaaz7715. doi:10.1126/scitranslmed.aaz7715. PMC 7610808 . PMID 32554708. S2CID 219722045.