The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Oxalyl chloride is an organic chemical compound with the formula (COCl)2. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
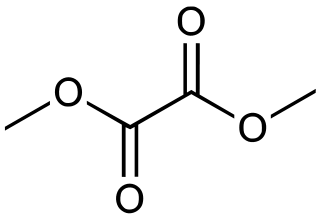
Dimethyl oxalate is an organic compound with the formula (CO2CH3)2. It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.

Peroxyoxalates are esters initially formed by the reaction of hydrogen peroxide with oxalate diesters or oxalyl chloride, with or without base, although the reaction is much faster with base:

Dodecahydroxycyclohexane is an organic compound with molecular formula C6O12H12 or C6(OH)12 or (C 2)6. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone.
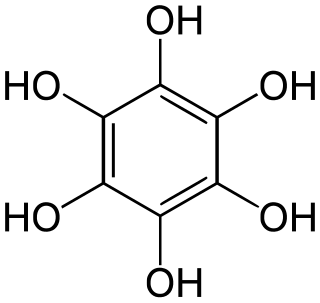
Benzenehexol, also called hexahydroxybenzene, is an organic compound with formula C
6H
6O
6 or C
6(OH)
6. It is a six-fold phenol of benzene. The product is also called hexaphenol, but this name has been used also for other substances.

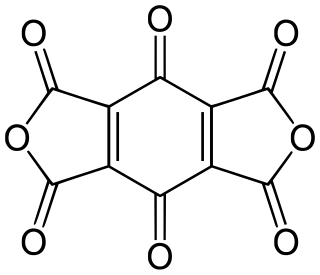
Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.

Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or para-benzoquinone.
2,5-Dihydroxy-1,4-benzoquinone or 2,5-dihydroxy-para-benzoquinone is an organic compound with formula C
6H
4O
4, formally derived from 1,4-benzoquinone by replacing two hydrogen atoms with hydroxyl (OH) groups. It is one of seven dihydroxybenzoquinone isomers. It is a yellow solid with planar molecules that exhibits ferroelectric properties.

Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C
6H
4O
3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.

Tetrahydroxy-1,4-benzoquinone bisoxalate is a chemical compound, an oxide of carbon with formula C
10O
10. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two oxalate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and oxalic acid.
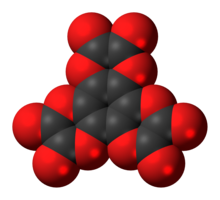
Benzoquinonetetracarboxylic dianhydride is an organic compound with formula C
10O
8 which can be seen as the result of removing two molecules of water H
2O from benzoquinonetetracarboxylic acid.
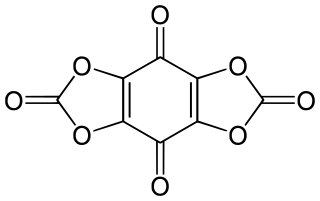
Tetrahydroxy-1,4-benzoquinone biscarbonate is a chemical compound, an oxide of carbon with formula C
8O
8. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two carbonate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and carbonic acid.

Hexahydroxybenzene triscarbonate is a chemical compound, an oxide of carbon with formula C
9O
9. Its molecular structure consists of a benzene core with the six hydrogen atoms replaced by three carbonate groups. It can be seen as a sixfold ester of hexahydroxybenzene (benzenehexol) and carbonic acid.

1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.
An yttrium compound is a chemical compound containing yttrium. Among these compounds, yttrium generally has a +3 valence. The solubility properties of yttrium compounds are similar to those of the lanthanides. For example oxalates and carbonates are hardly soluble in water, but soluble in excess oxalate or carbonate solutions as complexes are formed. Sulfates and double sulfates are generally soluble. They resemble the "yttrium group" of heavy lanthanide elements.
The carbonate oxalates are mixed anion compounds that contain both carbonate (CO3) and oxalate (C2O4) anions. Most compounds incorporate large trivalent metal ions, such as the rare earth elements. Some carbonate oxalate compounds of variable composition are formed by heating oxalates.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.