Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.
The nitrite ion has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid.
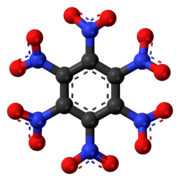
Octanitrocubane (molecular formula: C8(NO2)8) is a proposed high explosive that, like TNT, is shock-insensitive (not readily detonated by shock). The octanitrocubane molecule has the same chemical structure as cubane (C8H8) except that each of the eight hydrogen atoms is replaced by a nitro group (NO2). As of 1998, octanitrocubane had not been produced in quantities large enough to test its performance as an explosive.

Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 °C. Common hydrates are the hemipentahydrate and trihydrate.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Biphenyl is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen may use the prefixes xenyl or diphenylyl.

TATB, triaminotrinitrobenzene or 2,4,6-triamino-1,3,5-trinitrobenzene is an aromatic explosive, based on the basic six-carbon benzene ring structure with three nitro functional groups (NO2) and three amine (NH2) groups attached, alternating around the ring.

Sodium sulfide is a chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs.

1,3,5-Triazido-2,4,6-trinitrobenzene, also known as TATNB (triazidotrinitrobenzene) and TNTAZB (trinitrotriazidobenzene), is an aromatic high explosive composed of a benzene ring with three azido groups (-N3) and three nitro groups (-NO2) alternating around the ring, giving the chemical formula C6(N3)3(NO2)3. Its detonation velocity is 7,350 meters per second, which is comparable to TATB (triaminotrinitrobenzene).

Tetraphenylmethane is an organic compound consisting of a methane core with four phenyl substituents. It was first synthesized by Moses Gomberg in 1898.
Tetranitromethane or TNM is an organic oxidizer with chemical formula C(NO2)4. Its chemical structure consists of four nitro groups attached to one carbon atom. In 1857 it was first synthesised by the reaction of sodium cyanoacetamide with nitric acid.
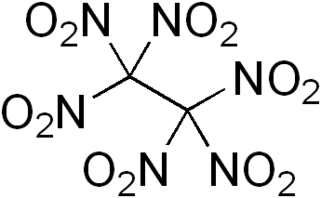
Hexanitroethane (HNE) is an organic compound with chemical formula C2N6O12 or (O2N)3C-C(NO2)3. It is a solid matter with a melting point of 135 °C.
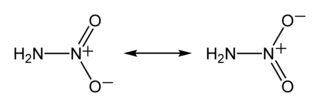
Nitramide or nitroamine is a chemical compound with the molecular formula H2N−NO2. Substituted derivatives R1R2N−NO2 are termed nitramides or nitroamines as well. Organyl derivatives of nitramide, R−NH−NO2 and R2N−NO2, are widely used as explosives: examples include RDX and HMX. It is an isomer of hyponitrous acid. Nitramide can be viewed as a nitrogen analog of nitric acid, in which the hydroxyl group −OH is replaced with the amino group −NH2.
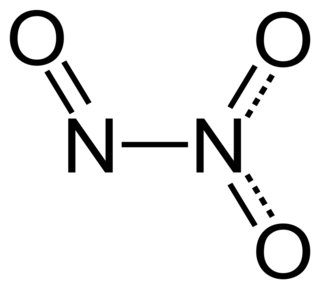
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):
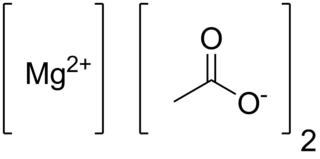
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2 and in its hydrated form, magnesium acetate tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium acetate is the magnesium salt of acetic acid. It is deliquescent and upon heating, it decomposes to form magnesium oxide. Magnesium acetate is commonly used as a source of magnesium in biological reactions.

Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.
2,4-Dinitroaniline is a chemical compound with a formula of C6H5N3O4. It is used as an explosive and as a reagent to detect and characterize aldehydes and ketones.