A drug test is a technical analysis of a biological specimen, for example urine, hair, blood, breath, sweat, and/or oral fluid/saliva—to determine the presence or absence of specified parent drugs or their metabolites. Major applications of drug testing include detection of the presence of performance enhancing steroids in sport, employers and parole/probation officers screening for drugs prohibited by law and police officers testing for the presence and concentration of alcohol (ethanol) in the blood commonly referred to as BAC. BAC tests are typically administered via a breathalyzer while urinalysis is used for the vast majority of drug testing in sports and the workplace. Numerous other methods with varying degrees of accuracy, sensitivity, and detection periods exist.

Cocaethylene (ethylbenzoylecgonine) is the ethyl ester of benzoylecgonine. It is structurally similar to cocaine, which is the methyl ester of benzoylecgonine. Cocaethylene is formed by the liver when cocaine and ethanol coexist in the blood. In 1885, cocaethylene was first synthesized, and in 1979, cocaethylene's side effects were discovered.

Fomepizole, also known as 4-methylpyrazole, is a medication used to treat methanol and ethylene glycol poisoning. It may be used alone or together with hemodialysis. It is given by injection into a vein.

Nordazepam is a 1,4-benzodiazepine derivative. Like other benzodiazepine derivatives, it has amnesic, anticonvulsant, anxiolytic, muscle relaxant, and sedative properties. However, it is used primarily in the treatment of anxiety disorders. It is an active metabolite of diazepam, chlordiazepoxide, clorazepate, prazepam, pinazepam, and medazepam.

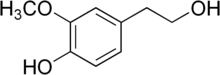
Homovanillic acid (HVA) is a major catecholamine metabolite that is produced by a consecutive action of monoamine oxidase and catechol-O-methyltransferase on dopamine. Homovanillic acid is used as a reagent to detect oxidative enzymes, and is associated with dopamine levels in the brain.

Silodosin is a medication for the symptomatic treatment of benign prostatic hyperplasia (BPH). It acts as an α1-adrenoceptor antagonist with high uroselectivity.

Metaclazepam is a drug which is a benzodiazepine derivative. It is a relatively selective anxiolytic with less sedative or muscle relaxant properties than other benzodiazepines such as diazepam or bromazepam. It has an active metabolite N-desmethylmetaclazepam, which is the main metabolite of metaclazepam. There is no significant difference in metabolism between younger and older individuals.
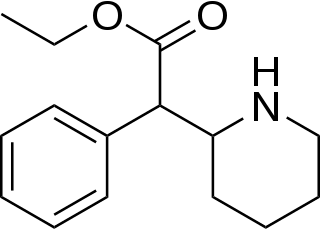
Ethylphenidate (EPH) is a psychostimulant and a close analog of methylphenidate.

Fluorenol is an alcohol derivative of fluorene. In the most significant isomer, fluoren-9-ol or 9-hydroxyfluorene, the hydroxy group is located on the bridging carbon between the two benzene rings. Hydroxyfluorene can be converted to fluorenone by oxidation. It is a white-cream colored solid at room temperature.
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable chemical compound (chemical formula CH3CHOHCH3) with a strong odor. As an isopropyl group linked to a hydroxyl group, it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of 1-propanol and ethyl methyl ether.

Ethyl glucuronide (EtG) is a metabolite of ethanol which is formed in the body by glucuronidation following exposure to ethanol, usually from drinking alcoholic beverages. It is used as a biomarker to test for ethanol use and to monitor alcohol abstinence in situations where drinking is prohibited, such as by the military, in alcohol treatment programs, in professional monitoring programs, in schools, liver transplant clinics, or in recovering alcoholic patients. In addition to its use to monitor abstinence and detect drinking, EtG also has potential for monitoring the amount of alcohol use over time because it can be detected in hair and nails, though the effectiveness of this has not yet been proven.
The molecular formula C9H12O3 (molar mass: 168.19 g/mol, exact mass: 168.078 u) may refer to:

Veratrole alcohol is an organic compound related to veratrole and also to benzyl alcohol. It can be obtained by reduction of veratraldehyde. Veratrole alcohol is the raw material for the synthesis of cyclotriveratrylene which is used in host–guest chemistry. It is a secondary metabolite of some white rot fungi and is believed to play a role in their degradation of lignin.

Drug–impaired driving, in the context of its legal definition, is the act of driving a motor vehicle while under the influence of an impairing substance. DUID, or Driving Under the Influence of Drugs, is prohibited in many countries. Several American states and European countries now have "per se" DUID laws that presume a driver is impaired if they are found to have any detectable quantity of controlled substances in their body while operating an automobile and that the driver has no doctor's prescription for the substance. This is similar to the "per se" DUI/DWI laws that presume a driver is impaired when their blood alcohol content is above a certain level. There is some controversy with "per se" DUID laws in that a driver with any detectable quantity of controlled substances may not in fact be impaired and the detectable quantity in blood or sweat may be only the remnants of drug use in days or weeks past. It is against road traffic safety.

Alcohol, sometimes referred to by the chemical name ethanol, is a psychoactive drug that is the active ingredient in drinks such as beer, wine, and distilled spirits. It is one of the oldest and most common recreational substances, causing the characteristic effects of alcohol intoxication ("drunkenness"). Among other effects, alcohol produces happiness and euphoria, decreased anxiety, increased sociability, sedation, impairment of cognitive, memory, motor, and sensory function, and generalized depression of central nervous system function. Ethanol is only one of several types of alcohol, but it is the only type of alcohol that is found in alcoholic beverages or commonly used for recreational purposes; other alcohols such as methanol and isopropyl alcohol are significantly more toxic. A mild, brief exposure to isopropanol, being only moderately more toxic than ethanol, is unlikely to cause any serious harm. Methanol, being profoundly more toxic than ethanol, is lethal in quantities as small as 10–15 milliliters.

Perillyl alcohol and its precursor limonene are naturally occurring monocyclic terpenes derived from the mevalonate pathway in plants. Perillyl alcohol can be found in the essential oils of various plants, such as lavender, lemongrass, sage, and peppermint. It has a number of manufacturing, household, and medical applications. For example, perillyl alcohol may be used as an ingredient in cleaning products and cosmetics.
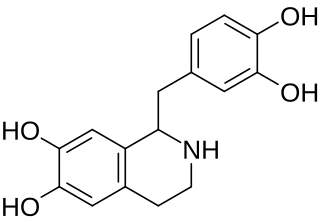
Tetrahydropapaveroline (norlaudanosoline) is a benzyltetrahydroisoquinoline alkaloid.

15β-Hydroxycyproterone acetate (15β-OH-CPA) is a steroidal antiandrogen and the major metabolite of cyproterone acetate (CPA). It is formed from CPA in the liver by hydroxylation via the cytochrome P450 enzyme CYP3A4. During therapy with CPA, 15β-OH-CPA circulates at concentrations that are approximately twice those of CPA. 15β-OH-CPA has similar or even greater antiandrogen activity compared to CPA. However, it has only about one-tenth of the activity of CPA as a progestogen. 15β-OH-CPA also shows some glucocorticoid activity, similarly to CPA and unesterified cyproterone.

A disulfiram-like drug is a drug that causes an adverse reaction to alcohol leading to nausea, vomiting, flushing, dizziness, throbbing headache, chest and abdominal discomfort, and general hangover-like symptoms among others. These effects are caused by accumulation of acetaldehyde, a major but toxic metabolite of alcohol formed by the enzyme alcohol dehydrogenase. The reaction has been variously termed a disulfiram-like reaction, alcohol intolerance, and acetaldehyde syndrome.
Two main questions arise in the law surrounding driving after having ingested cannabis: (1) whether cannabis actually impairs driving ability, and (2) whether the common practice of testing for THC is a reliable means to measure impairment. On the first question, studies are mixed. Several recent, extensive studies–including one conducted by the National Highway Traffic Safety Administration and one conducted by the American Automobile Association (AAA)–show that drivers with detectable THC in their blood are no more likely to cause car crashes than drivers with no amount of THC in their blood. Others show that cannabis can impair certain abilities important to safe driving –but no studies have been able to show that this increases the actual risk of crashing, or that drivers with THC in their blood cause a disproportionate number of crashes. On the second question, the studies that have been conducted so far have consistently found that THC blood levels and degree of impairment are not closely related. No known relationship between blood levels of THC and increased relative crash risk, or THC blood levels and level of driving impairment, has been shown by single-crash or classic-control studies. Thus, even though it is possible that cannabis impairs driving ability to some extent, there are currently no reliable means to test or measure whether a driver was actually impaired.