Abiotic stress is the negative impact of non-living factors on the living organisms in a specific environment. The non-living variable must influence the environment beyond its normal range of variation to adversely affect the population performance or individual physiology of the organism in a significant way.

Plant hormone are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, from embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and through to reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.

Abscisic acid (ABA) is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.

Inside a plant, the apoplast can mean the space outside of cell membranes, where material can diffuse freely; that is, the extracellular spaces. Apoplast can also refer especially to the continuum of cell walls of adjacent cells; fluid and material flows occurring there or in any extacellular space are called apoplastic flow or apoplastic transport.
Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure and matrix effects such as capillary action. The concept of water potential has proved useful in understanding and computing water movement within plants, animals, and soil. Water potential is typically expressed in potential energy per unit volume and very often is represented by the Greek letter ψ.

Hydrotropism is a plant's growth response in which the direction of growth is determined by a stimulus or gradient in water concentration. A common example is a plant root growing in humid air bending toward a higher relative humidity level.

In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution.
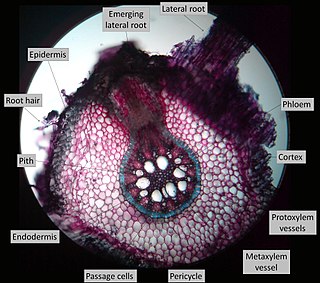
Lateral roots, emerging from the pericycle, extend horizontally from the primary root (radicle) and over time makeup the iconic branching pattern of root systems. They contribute to anchoring the plant securely into the soil, increasing water uptake, and facilitates the extraction of nutrients required for the growth and development of the plant. Lateral roots increase the surface area of a plant's root system and can be found in great abundance in several plant species. In some cases, lateral roots have been found to form symbiotic relationships with rhizobia (bacteria) and mycorrhizae (fungi) found in the soil, to further increase surface area and increase nutrient uptake.

Guard cells are specialized plant cells in the epidermis of leaves, stems and other organs that are used to control gas exchange. They are produced in pairs with a gap between them that forms a stomatal pore. The stomatal pores are largest when water is freely available and the guard cells turgid, and closed when water availability is critically low and the guard cells become flaccid. Photosynthesis depends on the diffusion of carbon dioxide (CO2) from the air through the stomata into the mesophyll tissues. Oxygen (O2), produced as a byproduct of photosynthesis, exits the plant via the stomata. When the stomata are open, water is lost by evaporation and must be replaced via the transpiration stream, with water taken up by the roots. Plants must balance the amount of CO2 absorbed from the air with the water loss through the stomatal pores, and this is achieved by both active and passive control of guard cell turgor pressure and stomatal pore size.
Drought tolerance is the ability to which a plant maintains its biomass production during arid or drought conditions. Some plants are naturally adapted to dry conditions, surviving with protection mechanisms such as desiccation tolerance, detoxification, or repair of xylem embolism. Other plants, specifically crops like corn, wheat, and rice, have become increasingly tolerant to drought with new varieties created via genetic engineering.

Phototropism is the growth of an organism in response to a light stimulus. Phototropism is most often observed in plants, but can also occur in other organisms such as fungi. The cells on the plant that are farthest from the light contain a hormone called auxin that reacts when phototropism occurs. This causes the plant to have elongated cells on the furthest side from the light. Phototropism is one of the many plant tropisms or movements which respond to external stimuli. Growth towards a light source is called positive phototropism, while growth away from light is called negative phototropism. Negative phototropism is not to be confused with skototropism which is defined as the growth towards darkness, whereas negative phototropism can refer to either the growth away from a light source or towards the darkness. Most plant shoots exhibit positive phototropism, and rearrange their chloroplasts in the leaves to maximize photosynthetic energy and promote growth. Some vine shoot tips exhibit negative phototropism, which allows them to grow towards dark, solid objects and climb them. The combination of phototropism and gravitropism allow plants to grow in the correct direction.
Stomatal conductance, usually measured in mmol m−2 s−1 by a porometer, estimates the rate of gas exchange and transpiration through the leaf stomata as determined by the degree of stomatal aperture.
A variation potential (VP) is a hydraulically propagating electrical signal occurring exclusively in plant cells. It is one of three propagating signals in plants, the other two being action potential (AP) and wound potential (WP). Variation potentials are responsible for the induction of many physiological processes and are a mechanism for plant systematic responses to local wounding. They induce changes in gene expression; the production of abscisic acid, jasmonic acid, and ethylene; temporary decreases in photosynthesis; and increases in respiration. Variation potentials have been widely shown in vascular plants.
Plants can be exposed to many stress factors such as disease, temperature changes, herbivory, injury and more. Therefore, in order to respond or be ready for any kind of physiological state, they need to develop some sort of system for their survival in the moment and/or for the future. Plant communication encompasses communication using volatile organic compounds, electrical signaling, and common mycorrhizal networks between plants and a host of other organisms such as soil microbes, other plants, animals, insects, and fungi. Plants communicate through a host of volatile organic compounds (VOCs) that can be separated into four broad categories, each the product of distinct chemical pathways: fatty acid derivatives, phenylpropanoids/benzenoids, amino acid derivatives, and terpenoids. Due to the physical/chemical constraints most VOCs are of low molecular mass, are hydrophobic, and have high vapor pressures. The responses of organisms to plant emitted VOCs varies from attracting the predator of a specific herbivore to reduce mechanical damage inflicted on the plant to the induction of chemical defenses of a neighboring plant before it is being attacked. In addition, the host of VOCs emitted varies from plant to plant, where for example, the Venus Fly Trap can emit VOCs to specifically target and attract starved prey. While these VOCs typically lead to increased resistance to herbivory in neighboring plants, there is no clear benefit to the emitting plant in helping nearby plants. As such, whether neighboring plants have evolved the capability to "eavesdrop" or whether there is an unknown tradeoff occurring is subject to much scientific debate. As related to the aspect of meaning-making, the field is also identified as phytosemiotics.

High Affinity K+ transporter HAK5 is a transport protein found on the cell surface membrane of plants under conditions of potassium deprivation. It is believed to act as a symporter for protons and the potassium ion, K+. Firstly discovered in barley, receiving the name of HvHAK1, it was soon after identified in the model plant Arabidopsis thaliana and named HAK5. These transporters belongs to the subgroup I of the KT-HAK-KUP family of plant proteins with obvious homology with both bacterial and fungal transport systems, which experienced a major diversification following land conquest. KT-HAK-KUP transporters are one of four different types of K+ transporter within the cell, but are unique as they do not have a putative pore forming domain like the other three; Shaker channels, KCO channels, HKT transporters. It is activated when the plant is situated in low soil with low potassium concentration, and has been shown to be located in higher concentration in the epidermis and vasculature of K+ deprived plants. By turning on, it increases the plants affinity (uptake) of potassium. Potassium plays a vital role in the plants growth, reproduction, immunity, ion homeostasis, and osmosis, which ensures the plants survival. It is the highest cationic molecule within the plant, accounting for 10% of the plants dry weight, which makes its uptake into the plant important. Each plant species has its own HAK5 transporter that is specific to that species and has different levels of affinity to K+. To operate and activate the HAK5 transporter, the external concentration of K+ must be lower than 10μM and up to 200μM. In Arabidopsis plants, when external potassium concentration is lower than 10μM, it is only HAK5 that is involved with the uptake of K+, then between 10 and 200μM both HAK5 and AKT1 are involved with the uptake of K+. HAK5 is coupled with CBL9/CIPK23 kinase's although the mechanism behind this has not yet been understood.
Feronia, also known as FER or protein Sirene, is a recognition receptor kinase found in plants. FER plays a significant part in the plant immune system as a receptor kinase which assists in immune signaling within plants, plant growth, and plant reproduction. FER is regulated by the Rapid Alkalinization Factor (RALF). FER regulates growth in normal environments but it is most beneficial in stressful environments as it helps to initiate immune signaling. FER can also play a role in reproduction in plants by participating in the communication between the female and male cells. FER is found in and can be studied in the organism Arabidopsis thaliana.
A cytokinin signaling and response regulator protein is a plant protein that is involved in a two step cytokinin signaling and response regulation pathway.
The P-type plasma membrane H+
-ATPase is found in plants and fungi. For the gastric H+
/K+
ATPase, see Hydrogen potassium ATPase.
As a model organism, the Arabidopsis thaliana response to salinity is studied to aid understanding of other more economically important crops.

Calcium signaling in Arabidopsis is a calcium mediated signalling pathway that Arabidopsis plants use in order to respond to a stimuli. In this pathway, Ca2+ works as a long range communication ion, allowing for rapid communication throughout the plant. Systemic changes in metabolites such as glucose and sucrose takes a few minutes after the stimulus, but gene transcription occurs within seconds. Because hormones, peptides and RNA travel through the vascular system at lower speeds than the plants response to wounds, indicates that Ca2+ must be involved in the rapid signal propagation. Instead of local communication to nearby cells and tissues, Ca2+ uses mass flow within the vascular system to help with rapid transport throughout the plant. Ca2+ moving through the xylem and phloem acts through a “calcium signature” receptor system in cells where they integrate the signal and respond with the activation of defense genes. These calcium signatures encode information about the stimulus allowing the response of the plant to cater towards the type of stimulus.









