Collagen is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Collagen makes up 30% of the protein found in the Human body. Vitamin E improves the production of collagen.

Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.

(2S,4R)-4-Hydroxyproline, or L-hydroxyproline (C5H9O3N), is an amino acid, abbreviated as Hyp or O, e.g., in Protein Data Bank.
In chemistry, hydroxylation can refer to:
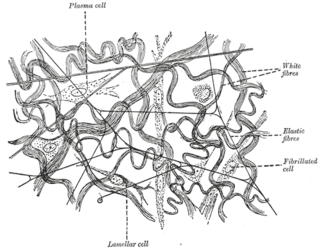
Elastic fibers are an essential component of the extracellular matrix composed of bundles of proteins (elastin) which are produced by a number of different cell types including fibroblasts, endothelial, smooth muscle, and airway epithelial cells. These fibers are able to stretch many times their length, and snap back to their original length when relaxed without loss of energy. Elastic fibers include elastin, elaunin and oxytalan.
Lysyl hydroxylases are alpha-ketoglutarate-dependent hydroxylases enzymes that catalyze the hydroxylation of lysine to hydroxylysine. Lysyl hydroxylases require iron and vitamin C as cofactors for their oxidation activity. It takes place following collagen synthesis in the cisternae (lumen) of the rough endoplasmic reticulum (ER). There are three lysyl hydroxylases (LH1-3) encoded in the human genome, namely: PLOD1, PLOD2 and PLOD3. From PLOD2 two splice variant can be expressed, where LH2b differs from LH2a by incorporating the small exon 13A. LH1 and LH3 hydroxylate lysyl residues in the collagen triple helix, whereas LH2b hydroxylates lysyl residues in the telopeptides of collagen. In addition to its hydroxylation activity, LH3 has glycosylation activity that produces either monosaccharide (Gal) or disaccharide (Glc-Gal) attached to collagen hydroxylysines.
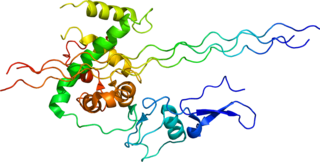
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.
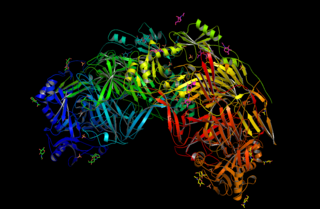
Lysyl oxidase (LOX), also known as protein-lysine 6-oxidase, is an enzyme that, in humans, is encoded by the LOX gene. It catalyzes the conversion of lysine residues into its aldehyde derivative allysine. Allysine form cross-links in extracellular matrix proteins. Inhibition of lysyl oxidase can cause osteolathyrism, but, at the same time, its upregulation by tumor cells may promote metastasis of the existing tumor, causing it to become malignant and cancerous.
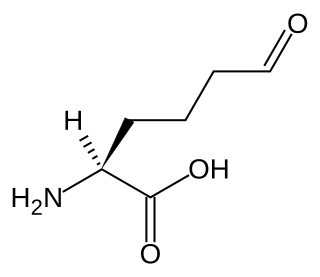
Allysine is a derivative of lysine that features a formyl group in place of the terminal amine. The free amino acid does not exist, but the allysine residue does. It is produced by aerobic oxidation of lysine residues by the enzyme lysyl oxidase. The transformation is an example of a post-translational modification. The semialdehyde form exists in equilibrium with a cyclic derivative.

In enzymology, a L-lysine 6-monooxygenase (NADPH) (EC 1.14.13.59) is an enzyme that catalyzes the chemical reaction

Procollagen-proline dioxygenase, commonly known as prolyl hydroxylase, is a member of the class of enzymes known as alpha-ketoglutarate-dependent hydroxylases. These enzymes catalyze the incorporation of oxygen into organic substrates through a mechanism that requires alpha-Ketoglutaric acid, Fe2+, and ascorbate. This particular enzyme catalyzes the formation of (2S, 4R)-4-hydroxyproline, a compound that represents the most prevalent post-translational modification in the human proteome.
In enzymology, an aerobactin synthase (EC 6.3.2.39) is an enzyme that catalyzes the chemical reaction
In enzymology, a lysine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a N6-hydroxylysine O-acetyltransferase (EC 2.3.1.102) is an enzyme that catalyzes the chemical reaction
In enzymology, a procollagen galactosyltransferase is an enzyme that catalyzes the chemical reaction

Procollagen-lysine,2-oxoglutarate 5-dioxygenase 3 is an enzyme that in humans is encoded by the PLOD3 gene.

Aerobactin is a bacterial iron chelating agent (siderophore) found in E. coli. It is a virulence factor enabling E. coli to sequester iron in iron-poor environments such as the urinary tract.

Donald Dexter Van Slyke, nicknamed Van, was a Dutch American biochemist. His achievements included the publication of 317 journal articles and 5 books, as well as numerous awards, among them the National Medal of Science and the first AMA Scientific Achievement Award. The Van Slyke determination, a test of amino acids, is named after him.
N2-citryl-N6-acetyl-N6-hydroxylysine synthase (EC 6.3.2.38, N(alpha)-citryl-N(epsilon)-acetyl-N(epsilon)-hydroxylysine synthase, iucA (gene)) is an enzyme with systematic name citrate:N6-acetyl-N6-hydroxy-L-lysine ligase (ADP-forming). This enzyme catalyses the following chemical reaction

Bruck syndrome is characterized as the combination of arthrogryposis multiplex congenita and osteogenesis imperfecta. Both diseases are uncommon, but concurrence is extremely rare which makes Bruck syndrome very difficult to research. Bruck syndrome is thought to be an atypical variant of osteogenesis imperfecta most resembling type III, if not its own disease. Multiple gene mutations associated with osteogenesis imperfecta are not seen in Bruck syndrome. Many affected individuals are within the same family, and pedigree data supports that the disease is acquired through autosomal recessive inheritance. Bruck syndrome has features of congenital contractures, bone fragility, recurring bone fractures, flexion joint and limb deformities, pterygia, short body height, and progressive kyphoscoliosis. Individuals encounter restricted mobility and pulmonary function. A reduction in bone mineral content and larger hydroxyapatite crystals are also detectable Joint contractures are primarily bilateral and symmetrical, and most prone to ankles. Bruck syndrome has no effect on intelligence, vision, or hearing.












