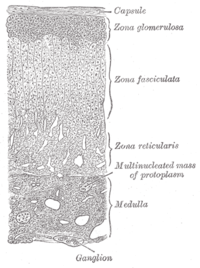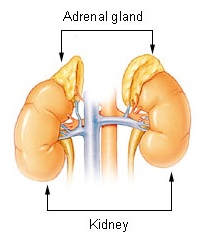
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.
Adrenocorticotropic hormone is a polypeptide tropic hormone produced by and secreted by the anterior pituitary gland. It is also used as a medication and diagnostic agent. ACTH is an important component of the hypothalamic-pituitary-adrenal axis and is often produced in response to biological stress. Its principal effects are increased production and release of cortisol by the cortex of the adrenal gland. ACTH is also related to the circadian rhythm in many organisms.

Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks, a round red face, a fat lump between the shoulders, weak muscles, weak bones, acne, and fragile skin that heals poorly. Women may have more hair and irregular menstruation. Occasionally there may be changes in mood, headaches, and a chronic feeling of tiredness.

The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.

Cortisol is a steroid hormone, in the glucocorticoid class of hormones. When used as a medication, it is known as hydrocortisone.

Addison's disease, also known as primary adrenal insufficiency, is a rare long-term endocrine disorder characterized by inadequate production of the steroid hormones cortisol and aldosterone by the two outer layers of the cells of the adrenal glands, causing adrenal insufficiency. Symptoms generally come on slowly and insidiously and may include abdominal pain and gastrointestinal abnormalities, weakness, and weight loss. Darkening of the skin in certain areas may also occur. Under certain circumstances, an adrenal crisis may occur with low blood pressure, vomiting, lower back pain, and loss of consciousness. Mood changes may also occur. Rapid onset of symptoms indicates acute adrenal failure, which is a clinical emergency. An adrenal crisis can be triggered by stress, such as from an injury, surgery, or infection.

Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.

Adrenal insufficiency is a condition in which the adrenal glands do not produce adequate amounts of steroid hormones. The adrenal gland normally secretes glucocorticoids, mineralocorticoids, and androgens. These hormones are important in regulating blood pressure, electrolytes, and metabolism as a whole. Deficiency of these hormones leads to symptoms ranging from abdominal pain, vomiting, muscle weakness and fatigue, low blood pressure, depression, mood and personality changes to organ failure and shock. An adrenal crisis may occur if the body is subjected to stress, such as an accident, injury, surgery, or severe infection; this is a life-threatening medical condition resulting from severe deficiency of cortisol in the body. Death may quickly follow.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.

Lipoid congenital adrenal hyperplasia is an endocrine disorder that is an uncommon and potentially lethal form of congenital adrenal hyperplasia (CAH). It arises from defects in the earliest stages of steroid hormone synthesis: the transport of cholesterol into the mitochondria and the conversion of cholesterol to pregnenolone—the first step in the synthesis of all steroid hormones. Lipoid CAH causes mineralocorticoid deficiency in affected infants and children. Male infants are severely undervirilized causing their external genitalia to look feminine. The adrenals are large and filled with lipid globules derived from cholesterol.

Hypoaldosteronism is an endocrinological disorder characterized by decreased levels of the hormone aldosterone. Similarly, isolated hypoaldosteronism is the condition of having lowered aldosterone without corresponding changes in cortisol.
Secondary hypertension is a type of hypertension which by definition is caused by an identifiable underlying primary cause. It is much less common than the other type, called essential hypertension, affecting only 5-10% of hypertensive patients. It has many different causes including endocrine diseases, kidney diseases, and tumors. It also can be a side effect of many medications.
In humans and other animals, the adrenocortical hormones are hormones produced by the adrenal cortex, the outer region of the adrenal gland. These polycyclic steroid hormones have a variety of roles that are crucial for the body’s response to stress, and they also regulate other functions in the body. Threats to homeostasis, such as injury, chemical imbalances, infection, or psychological stress, can initiate a stress response. Examples of adrenocortical hormones that are involved in the stress response are aldosterone and cortisol. These hormones also function in regulating the conservation of water by the kidneys and glucose metabolism, respectively.
The ACTH test is a medical test usually requested and interpreted by endocrinologists to assess the functioning of the adrenal glands' stress response by measuring the adrenal response to adrenocorticotropic hormone or another corticotropic agent such as tetracosactide or alsactide (Synchrodyn). ACTH is a hormone produced in the anterior pituitary gland that stimulates the adrenal glands to release cortisol, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and aldosterone.

Adrenal crisis is a potentially life-threatening medical condition requiring immediate emergency treatment. It is a constellation of symptoms that indicate severe adrenal insufficiency. This may be the result of either previously undiagnosed or untreated Addison's disease, a disease process suddenly affecting adrenal function, suddenly stopping intake of glucocorticoids or an intercurrent problem in someone known to have Addison's disease, congenital adrenal hyperplasia (CAH), or other form of primary adrenal insufficiency.
Critical illness–related corticosteroid insufficiency is a form of adrenal insufficiency in critically ill patients who have blood corticosteroid levels which are inadequate for the severe stress response they experience. Combined with decreased glucocorticoid receptor sensitivity and tissue response to corticosteroids, this adrenal insufficiency constitutes a negative prognostic factor for intensive care patients.
Adrenal gland disorders are conditions that interfere with the normal functioning of the adrenal glands. Adrenal disorders may cause hyperfunction or hypofunction, and may be congenital or acquired.
Glucocorticoid remediable aldosteronism also describable as aldosterone synthase hyperactivity, is an autosomal dominant disorder in which the increase in aldosterone secretion produced by ACTH is no longer transient.

Adrenocorticotropic hormone is used as a medication and as diagnostic agent in the ACTH stimulation test.
Adrenal steroids are steroids that are derived from the adrenal glands. They include corticosteroids, which consist of glucocorticoids like cortisol and mineralocorticoids like aldosterone, adrenal androgens like dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), and androstenedione (A4), and neurosteroids like DHEA and DHEA-S, as well as pregnenolone and pregnenolone sulfate (P5-S). Adrenal steroids are specifically produced in the adrenal cortex.