An immune response is a physiological reaction which occurs within an organism in the context of inflammation for the purpose of defending against exogenous factors. These include a wide variety of different toxins, viruses, intra- and extracellular bacteria, protozoa, helminths, and fungi which could cause serious problems to the health of the host organism if not cleared from the body.

Phagocytosis is the process by which a cell uses its plasma membrane to engulf a large particle, giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte.
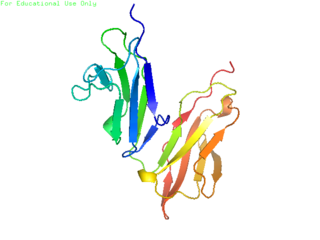
CD32, also known as FcγRII or FCGR2, is a surface receptor glycoprotein belonging to the Ig gene superfamily. CD32 can be found on the surface of a variety of immune cells. CD32 has a low-affinity for the Fc region of IgG antibodies in monomeric form, but high affinity for IgG immune complexes. CD32 has two major functions: cellular response regulation, and the uptake of immune complexes. Cellular responses regulated by CD32 include phagocytosis, cytokine stimulation, and endocytic transport. Dysregulated CD32 is associated with different forms of autoimmunity, including systemic lupus erythematosus. In humans, there are three major CD32 subtypes: CD32A, CD32B, and CD32C. While CD32A and CD32C are involved in activating cellular responses, CD32B is inhibitory.
Humoral immunity is the aspect of immunity that is mediated by macromolecules – including secreted antibodies, complement proteins, and certain antimicrobial peptides – located in extracellular fluids. Humoral immunity is named so because it involves substances found in the humors, or body fluids. It contrasts with cell-mediated immunity. Humoral immunity is also referred to as antibody-mediated immunity.
Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus, opsonins act as tags to label things in the body that should be phagocytosed by phagocytes. Different types of things ("targets") can be tagged by opsonins for phagocytosis, including: pathogens, cancer cells, aged cells, dead or dying cells, excess synapses, or protein aggregates. Opsonins help clear pathogens, as well as dead, dying and diseased cells.

The adaptive immune system, also known as the acquired immune system, or specific immune system is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system is one of the two main immunity strategies found in vertebrates.

An antigen-presenting cell (APC) or accessory cell is a cell that displays an antigen bound by major histocompatibility complex (MHC) proteins on its surface; this process is known as antigen presentation. T cells may recognize these complexes using their T cell receptors (TCRs). APCs process antigens and present them to T cells.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.

Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.

In immunology, an Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.

The B-cell receptor (BCR) is a transmembrane protein on the surface of a B cell. A B-cell receptor is composed of a membrane-bound immunoglobulin molecule and a signal transduction moiety. The former forms a type 1 transmembrane receptor protein, and is typically located on the outer surface of these lymphocyte cells. Through biochemical signaling and by physically acquiring antigens from the immune synapses, the BCR controls the activation of the B cell. B cells are able to gather and grab antigens by engaging biochemical modules for receptor clustering, cell spreading, generation of pulling forces, and receptor transport, which eventually culminates in endocytosis and antigen presentation. B cells' mechanical activity adheres to a pattern of negative and positive feedbacks that regulate the quantity of removed antigen by manipulating the dynamic of BCR–antigen bonds directly. Particularly, grouping and spreading increase the relation of antigen with BCR, thereby proving sensitivity and amplification. On the other hand, pulling forces delinks the antigen from the BCR, thus testing the quality of antigen binding.

An alveolar macrophage, pulmonary macrophage, is a type of macrophage, a professional phagocyte, found in the airways and at the level of the alveoli in the lungs, but separated from their walls.

C3b is the larger of two elements formed by the cleavage of complement component 3, and is considered an important part of the innate immune system. C3b is potent in opsonization: tagging pathogens, immune complexes (antigen-antibody), and apoptotic cells for phagocytosis. Additionally, C3b plays a role in forming a C3 convertase when bound to Factor B, or a C5 convertase when bound to C4b and C2b or when an additional C3b molecule binds to the C3bBb complex.
CD64 is a type of integral membrane glycoprotein known as an Fc receptor that binds monomeric IgG-type antibodies with high affinity. It is more commonly known as Fc-gamma receptor 1 (FcγRI). After binding IgG, CD64 interacts with an accessory chain known as the common γ chain, which possesses an ITAM motif that is necessary for triggering cellular activation.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.

Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication. The suboptimal antibodies can result from natural infection or from vaccination. ADE may cause enhanced respiratory disease, but is not limited to respiratory disease. It has been observed in HIV, RSV virus and Dengue virus and is monitored for in vaccine development.
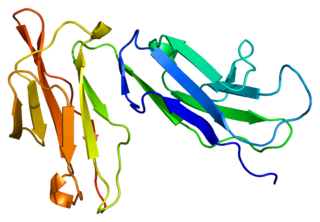
Fc fragment of IgG receptor IIb is a low affinity inhibitory receptor for the Fc region of immunoglobulin gamma (IgG). FCGR2B participates in the phagocytosis of immune complexes and in the regulation of antibody production by B lymphocytes.

Fc fragment of IgA receptor (FCAR) is a human gene that codes for the transmembrane receptor FcαRI, also known as CD89. FcαRI binds the heavy-chain constant region of Immunoglobulin A (IgA) antibodies. FcαRI is present on the cell surface of myeloid lineage cells, including neutrophils, monocytes, macrophages, and eosinophils, though it is notably absent from intestinal macrophages and does not appear on mast cells. FcαRI plays a role in both pro- and anti-inflammatory responses depending on the state of IgA bound. Inside-out signaling primes FcαRI in order for it to bind its ligand, while outside-in signaling caused by ligand binding depends on FcαRI association with the Fc receptor gamma chain.
The following outline is provided as an overview of and topical guide to immunology:














