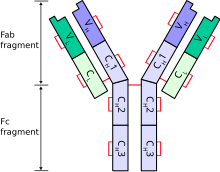
An antibody (Ab) is the secreted form of a B cell receptor; the term immunoglobulin (Ig) can refer to either the membrane-bound form or the secreted form of the B cell receptor, but they are, broadly speaking, the same protein, and so the terms are often treated as synonymous. Antibodies are large, Y-shaped proteins belonging to the immunoglobulin superfamily which are used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that cause disease. Antibodies can recognize virtually any size antigen with diverse chemical compositions from molecules. Each antibody recognizes one or more specific antigens. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each tip of the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

Immunoglobulin G (IgG) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG antibody has two paratopes.

Immunoglobulin A is an antibody that plays a role in the immune function of mucous membranes. The amount of IgA produced in association with mucosal membranes is greater than all other types of antibody combined. In absolute terms, between three and five grams are secreted into the intestinal lumen each day. This represents up to 15% of total immunoglobulins produced throughout the body.
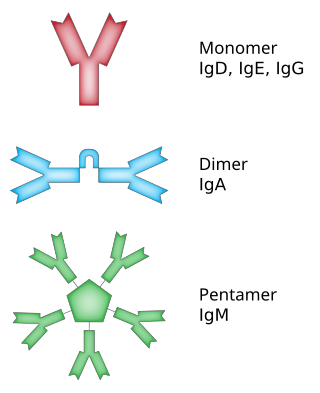
Immunoglobulin D (IgD) is an antibody isotype that makes up about 1% of proteins in the plasma membranes of immature B-lymphocytes where it is usually co-expressed with another cell surface antibody called IgM. IgD is also produced in a secreted form that is found in very small amounts in blood serum, representing 0.25% of immunoglobulins in serum. The relative molecular mass and half-life of secreted IgD is 185 kDa and 2.8 days, respectively. Secreted IgD is produced as a monomeric antibody with two heavy chains of the delta (δ) class, and two Ig light chains.
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.
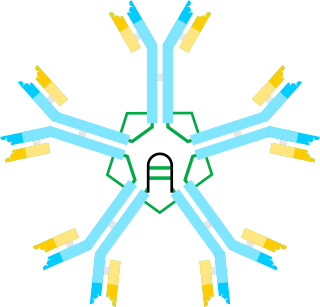
Immunoglobulin M (IgM) is the largest of several isotypes of antibodies that are produced by vertebrates. IgM is the first antibody to appear in the response to initial exposure to an antigen; causing it to also be called an acute phase antibody. In humans and other mammals that have been studied, plasmablasts in the spleen are the main source of specific IgM production.

A single-domain antibody (sdAb), also known as a Nanobody, is an antibody fragment consisting of a single monomeric variable antibody domain. Like a whole antibody, it is able to bind selectively to a specific antigen. With a molecular weight of only 12–15 kDa, single-domain antibodies are much smaller than common antibodies which are composed of two heavy protein chains and two light chains, and even smaller than Fab fragments and single-chain variable fragments.
V(D)J recombination is the mechanism of somatic recombination that occurs only in developing lymphocytes during the early stages of T and B cell maturation. It results in the highly diverse repertoire of antibodies/immunoglobulins and T cell receptors (TCRs) found in B cells and T cells, respectively. The process is a defining feature of the adaptive immune system.

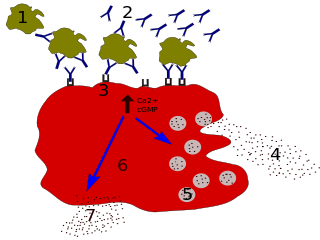
The B-cell receptor (BCR) is a transmembrane protein on the surface of a B cell. A B-cell receptor is composed of a membrane-bound immunoglobulin molecule and a signal transduction moiety. The former forms a type 1 transmembrane receptor protein, and is typically located on the outer surface of these lymphocyte cells. Through biochemical signaling and by physically acquiring antigens from the immune synapses, the BCR controls the activation of the B cell. B cells are able to gather and grab antigens by engaging biochemical modules for receptor clustering, cell spreading, generation of pulling forces, and receptor transport, which eventually culminates in endocytosis and antigen presentation. B cells' mechanical activity adheres to a pattern of negative and positive feedbacks that regulate the quantity of removed antigen by manipulating the dynamic of BCR–antigen bonds directly. Particularly, grouping and spreading increase the relation of antigen with BCR, thereby proving sensitivity and amplification. On the other hand, pulling forces delinks the antigen from the BCR, thus testing the quality of antigen binding.

Complementarity-determining regions (CDRs) are polypeptide segments of the variable chains in immunoglobulins (antibodies) and T cell receptors, generated by B-cells and T-cells respectively. CDRs are where these molecules bind to their specific antigen and their structure/sequence determines the binding activity of the respective antibody. A set of CDRs constitutes a paratope, or the antigen-binding site. As the most variable parts of the molecules, CDRs are crucial to the diversity of antigen specificities generated by lymphocytes.

Immunoglobulin class switching, also known as isotype switching, isotypic commutation or class-switch recombination (CSR), is a biological mechanism that changes a B cell's production of immunoglobulin from one type to another, such as from the isotype IgM to the isotype IgG. During this process, the constant-region portion of the antibody heavy chain is changed, but the variable region of the heavy chain stays the same. Since the variable region does not change, class switching does not affect antigen specificity. Instead, the antibody retains affinity for the same antigens, but can interact with different effector molecules.

The immunoglobulin light chain is the small polypeptide subunit of an antibody (immunoglobulin).

In immunology, antibodies are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen . In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen . They appear at different stages of an immune response, differ in structural features, and in their location around the body.

The Joining (J) chain is a protein component that links monomers of antibodies IgM and IgA to form polymeric antibodies capable of secretion. The J chain is well conserved in the animal kingdom, but its specific functions are yet to be fully understood. It is a 137 residue polypeptide, encoded by the IGJ gene.

Ig epsilon chain C region is a protein that in humans is encoded by the IGHE gene.

Cluster of differentiation CD79A also known as B-cell antigen receptor complex-associated protein alpha chain and MB-1 membrane glycoprotein, is a protein that in humans is encoded by the CD79A gene.

Humoral immune deficiencies are conditions which cause impairment of humoral immunity, which can lead to immunodeficiency. It can be mediated by insufficient number or function of B cells, the plasma cells they differentiate into, or the antibody secreted by the plasma cells. The most common such immunodeficiency is inherited selective IgA deficiency, occurring between 1 in 100 and 1 in 1000 persons, depending on population. They are associated with increased vulnerability to infection, but can be difficult to detect in the absence of infection.
A heavy-chain antibody is an antibody which consists only of two heavy chains and lacks the two light chains usually found in antibodies.
Antibody structure is made up of two heavy-chains and two light-chains. These chains are held together by disulfide bonds. The arrangement or processes that put together different parts of this antibody molecule play important role in antibody diversity and production of different subclasses or classes of antibodies. The organization and processes take place during the development and differentiation of B cells. That is, the controlled gene expression during transcription and translation coupled with the rearrangements of immunoglobulin gene segments result in the generation of antibody repertoire during development and maturation of B cells.
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity.