Related Research Articles
The cluster of differentiation is a protocol used for the identification and investigation of cell surface molecules providing targets for immunophenotyping of cells. In terms of physiology, CD molecules can act in numerous ways, often acting as receptors or ligands important to the cell. A signal cascade is usually initiated, altering the behavior of the cell. Some CD proteins do not play a role in cell signaling, but have other functions, such as cell adhesion. CD for humans is numbered up to 371.

A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells, T cells, and B cells. They are the main type of cell found in lymph, which prompted the name "lymphocyte". Lymphocytes make up between 18% and 42% of circulating white blood cells.

Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte. They are responsible for inflammatory reactions during immune response, as well as in the formation of acute and chronic allergic diseases, including anaphylaxis, asthma, atopic dermatitis and hay fever. They also produce compounds that coordinate immune responses, including histamine and serotonin that induce inflammation, heparin that prevents blood clotting, although there are less than that found in mast cell granules. Mast cells were once thought to be basophils that migrated from blood into their resident tissues, but they are now known to be different types of cells.

Flow cytometry (FC) is a technique used to detect and measure physical and chemical characteristics of a population of cells or particles.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.
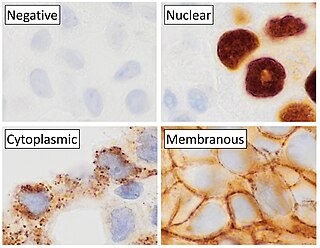
Immunohistochemistry (IHC) is the most common application of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue. Albert Coons conceptualized and first implemented the procedure in 1941.

Hybridoma technology is a method for producing large numbers of identical antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal B cell cancer cells, a myeloma, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma. The hybridomas can be grown in culture, each culture starting with one viable hybridoma cell, producing cultures each of which consists of genetically identical hybridomas which produce one antibody per culture (monoclonal) rather than mixtures of different antibodies (polyclonal). The myeloma cell line that is used in this process is selected for its ability to grow in tissue culture and for an absence of antibody synthesis. In contrast to polyclonal antibodies, which are mixtures of many different antibody molecules, the monoclonal antibodies produced by each hybridoma line are all chemically identical.

Carcinoembryonic antigen (CEA) describes a set of highly related glycoproteins involved in cell adhesion. CEA is normally produced in gastrointestinal tissue during fetal development, but the production stops before birth. Consequently, CEA is usually present at very low levels in the blood of healthy adults. However, the serum levels are raised in some types of cancer, which means that it can be used as a tumor marker in clinical tests. Serum levels can also be elevated in heavy smokers.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
Immunomagnetic separation (IMS) is a laboratory tool that can efficiently isolate cells out of body fluid or cultured cells. It can also be used as a method of quantifying the pathogenicity of food, blood or feces. DNA analysis have supported the combined use of both this technique and Polymerase Chain Reaction (PCR). Another laboratory separation tool is the affinity magnetic separation (AMS), which is more suitable for the isolation of prokaryotic cells.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.

An antibody microarray is a specific form of protein microarray. In this technology, a collection of captured antibodies are spotted and fixed on a solid surface such as glass, plastic, membrane, or silicon chip, and the interaction between the antibody and its target antigen is detected. Antibody microarrays are often used for detecting protein expression from various biofluids including serum, plasma and cell or tissue lysates. Antibody arrays may be used for both basic research and medical and diagnostic applications.
Flow-FISH is a cytogenetic technique to quantify the copy number of RNA or specific repetitive elements in genomic DNA of whole cell populations via the combination of flow cytometry with cytogenetic fluorescent in situ hybridization staining protocols.

Allergic symptoms are caused by an initial systemic histamine release by activated basophils and mast cells, that may lead to shock with laryngeal edema, lower-airway obstruction and hypotension. This is why basophils are considered with mast cells to be the key cells in allergic diseases.
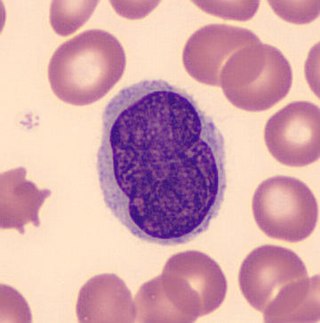
Lutzner cells were discovered by Marvin A. Lutzner, Lucien-Marie Pautrier, and Albert Sézary. These cells are described as the smaller forms of Sézary cells, or Sézary-Lutzner cells, and the two variants are recognised as being morphologically different. Aggregates of these cells in mycosis fungoides are known as a Pautrier's microabscesses. They are a form of T-lymphocytes that has been mutated This atypical form of T-lymphocytes contains T-cell receptors on the surface and is found in both the dermis and epidermis layers of the skin. Since Lutzner cells are a mutated form of T-lymphocytes, they develop in bone marrow and are transported to the thymus is order to mature. The production and maturation stages occur before the cell has developed a mutation. Lutzner cells can form cutaneous T-cell lymphoma, which is a form of skin cancer.

Proximity ligation assay is a technology that extends the capabilities of traditional immunoassays to include direct detection of proteins, protein interactions, Extracellular vesicles and post translational modifications with high specificity and sensitivity. Protein targets can be readily detected and localized with single molecule resolution and objectively quantified in unmodified cells and tissues. Utilizing only a few cells, sub-cellular events, even transient or weak interactions, are revealed in situ and sub-populations of cells can be differentiated. Within hours, results from conventional co-immunoprecipitation and co-localization techniques can be confirmed.
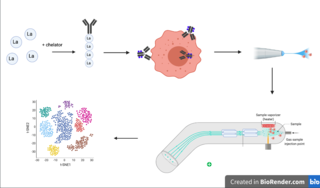
Cytometry by time of flight, or CyTOF, is an application of mass cytometry used to quantify labeled targets on the surface and interior of single cells. CyTOF allows the quantification of multiple cellular components simultaneously using an ICP-MS detector.

Mass cytometry is a mass spectrometry technique based on inductively coupled plasma mass spectrometry and time of flight mass spectrometry used for the determination of the properties of cells (cytometry). In this approach, antibodies are conjugated with isotopically pure elements, and these antibodies are used to label cellular proteins. Cells are nebulized and sent through an argon plasma, which ionizes the metal-conjugated antibodies. The metal signals are then analyzed by a time-of-flight mass spectrometer. The approach overcomes limitations of spectral overlap in flow cytometry by utilizing discrete isotopes as a reporter system instead of traditional fluorophores which have broad emission spectra.

In cell biology, single-cell variability occurs when individual cells in an otherwise similar population differ in shape, size, position in the cell cycle, or molecular-level characteristics. Such differences can be detected using modern single-cell analysis techniques. Investigation of variability within a population of cells contributes to understanding of developmental and pathological processes,
Tissue image cytometry or tissue cytometry is a method of digital histopathology and combines classical digital pathology and computational pathology into one integrated approach with solutions for all kinds of diseases, tissue and cell types as well as molecular markers and corresponding staining methods to visualize these markers. Tissue cytometry uses virtual slides as they can be generated by multiple, commercially available slide scanners, as well as dedicated image analysis software – preferentially including machine and deep learning algorithms. Tissue cytometry enables cellular analysis within thick tissues, retaining morphological and contextual information, including spatial information on defined cellular subpopulations. In this process, a tissue sample, either formalin-fixed paraffin-embedded (FFPE) or frozen tissue section, also referred to as “cryocut”, is labelled with either immunohistochemistry(IHC) or immunofluorescent markers, scanned with high-throughput slide scanners and the data gathered from virtual slides is processed and analyzed using software that is able to identify individual cells in tissue context automatically and distinguish between nucleus and cytoplasm for each cell. Additional algorithms can identify cellular membranes, subcellular structures and/or multicellular tissue structures.
References
- ↑ Young, Yoon Kow; Bolt, Alicia M.; Ahn, Ryuhjin; Mann, Koren K. (2016). "Analyzing the Tumor Microenvironment by Flow Cytometry". Methods in Molecular Biology . 1458: 95–110. doi:10.1007/978-1-4939-3801-8_8. ISSN 1940-6029. PMID 27581017.