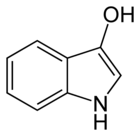
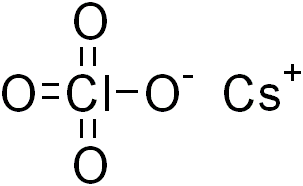Indigo dye is an organic compound with a distinctive blue color. Indigo is a natural dye extracted from the leaves of some plants of the Indigofera genus, in particular Indigofera tinctoria; dye-bearing Indigofera plants were commonly grown and used throughout the world, in Asia in particular, as an important crop, with the production of indigo dyestuff economically important due to the historical rarity of other blue dyestuffs.

Maltose, also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar.

Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as N-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin.
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound N,N-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide. Acetamide can be considered an intermediate between acetone, which has two methyl (CH3) groups either side of the carbonyl (CO), and urea which has two amide (NH2) groups in those locations. Acetamide is also a naturally occurring mineral with the IMA symbol: Ace.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipenahydrate CdCl2•2.5H2O.
This page provides supplementary chemical data on ethanol.
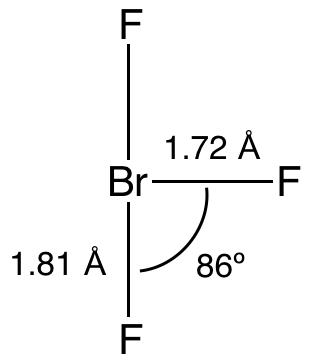
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.
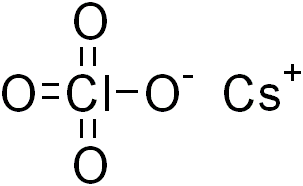
Caesium perchlorate or cesium perchlorate (CsClO4), is a perchlorate of caesium. It forms white crystals, which are sparingly soluble in cold water and ethanol. It dissolves more easily in hot water.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.
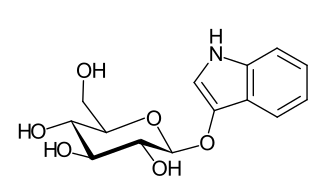
Indican is a colourless organic compound, soluble in water, naturally occurring in Indigofera plants. It is a precursor of indigo dye.

Hydrogen telluride is the inorganic compound with the formula H2Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist at very low concentrations long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.

Henry Edward Schunck, also known as Edward von Schunck, was a British chemist who did much work with dyes.

Mercury(I) fluoride or mercurous fluoride is the chemical compound composed of mercury and fluorine with the formula Hg2F2. It consists of small yellow cubic crystals, which turn black when exposed to light.

Holmium(III) chloride is the inorganic compound with the formula HoCl3. It is a common salt but is mainly used in research. It can be used to produce pure holmium. It exhibits the same color-changing behavior seen in holmium oxide, being a yellow in natural lighting and a bright pink color in fluorescent lighting.

2-Pentanol is an organic chemical compound. It is used as a solvent and an intermediate in the manufacturing of other chemicals. 2-Pentanol is a component of many mixtures of amyl alcohols sold industrially. 2-Pentanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-2-pentanol and (S)-(+)-2-pentanol.

Benzyl iodide is an organic compound with the chemical formula C
7H
7I. The compound consists of a benzene ring with an attached iodidemethyl group. The substance is an alkyl halide and is a constitutional isomer of the iodotoluenes.

1,9-Nonanediol, also known as nonamethylene glycol, is a diol with the molecular formula HO(CH2)9OH. It is a colorless solid, which is sparingly soluble in water but readily soluble in ethanol.