Related Research Articles

Polycystic ovary syndrome, or polycystic ovarian syndrome (PCOS), is the most common endocrine disorder in women of reproductive age. The syndrome is named after cysts which form on the ovaries of some people with this condition, though this is not a universal symptom, and not the underlying cause of the disorder.

Ovulation is the release of eggs from the ovaries. In women, this event occurs when the ovarian follicles rupture and release the secondary oocyte ovarian cells. After ovulation, during the luteal phase, the egg will be available to be fertilized by sperm. In addition, the uterine lining (endometrium) is thickened to be able to receive a fertilized egg. If no conception occurs, the uterine lining as well as the egg will be shed during menstruation.
Amenorrhea is the absence of a menstrual period in a female who has reached reproductive age. Physiological states of amenorrhoea are seen, most commonly, during pregnancy and lactation (breastfeeding). Outside the reproductive years, there is absence of menses during childhood and after menopause.
Anovulation is when the ovaries do not release an oocyte during a menstrual cycle. Therefore, ovulation does not take place. However, a woman who does not ovulate at each menstrual cycle is not necessarily going through menopause. Chronic anovulation is a common cause of infertility.

Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome. Use results in a greater chance of twins. It is taken by mouth once a day, with a course of treatment that usually lasts for five days.

Hyperandrogenism is a medical condition characterized by high levels of androgens. It is more common in women than men. Symptoms of hyperandrogenism may include acne, seborrhea, hair loss on the scalp, increased body or facial hair, and infrequent or absent menstruation. Complications may include high blood cholesterol and diabetes. It occurs in approximately 5% of women of reproductive age.
An anovulatory cycle is a menstrual cycle characterised by the absence of ovulation and a luteal phase. It may also vary in duration from a regular menstrual cycle.
Fertility medications, also known as fertility drugs, are medications which enhance reproductive fertility. For women, fertility medication is used to stimulate follicle development of the ovary. There are very few fertility medication options available for men.

Anti-Müllerian hormone (AMH), also known as Müllerian-inhibiting hormone (MIH), is a glycoprotein hormone structurally related to inhibin and activin from the transforming growth factor beta superfamily, whose key roles are in growth differentiation and folliculogenesis. In humans, it is encoded by the AMH gene, on chromosome 19p13.3, while its receptor is encoded by the AMHR2 gene on chromosome 12.

The hypothalamic–pituitary–gonadal axis refers to the hypothalamus, pituitary gland, and gonadal glands as if these individual endocrine glands were a single entity. Because these glands often act in concert, physiologists and endocrinologists find it convenient and descriptive to speak of them as a single system.

Ovarian reserve is a term that is used to determine the capacity of the ovary to provide egg cells that are capable of fertilization resulting in a healthy and successful pregnancy. With advanced maternal age the number of egg cell that can be successfully recruited for a possible pregnancy declines, constituting a major factor in the inverse correlation between age and female fertility.
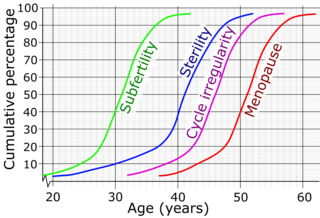
Female infertility refers to infertility in women. It affects an estimated 48 million women, with the highest prevalence of infertility affecting women in South Asia, Sub-Saharan Africa, North Africa/Middle East, and Central/Eastern Europe and Central Asia. Infertility is caused by many sources, including nutrition, diseases, and other malformations of the uterus. Infertility affects women from around the world, and the cultural and social stigma surrounding it varies.
Ovulation induction is the stimulation of ovulation by medication. It is usually used in the sense of stimulation of the development of ovarian follicles to reverse anovulation or oligoovulation.
Controlled ovarian hyperstimulation is a technique used in assisted reproduction involving the use of fertility medications to induce ovulation by multiple ovarian follicles. These multiple follicles can be taken out by oocyte retrieval for use in in vitro fertilisation (IVF), or be given time to ovulate, resulting in superovulation which is the ovulation of a larger-than-normal number of eggs, generally in the sense of at least two. When ovulated follicles are fertilised in vivo, whether by natural or artificial insemination, there is a very high risk of a multiple pregnancy.
Poor ovarian reserve is a condition of low fertility characterized by 1): low numbers of remaining oocytes in the ovaries or 2) possibly impaired preantral oocyte development or recruitment. Recent research suggests that premature ovarian aging and premature ovarian failure may represent a continuum of premature ovarian senescence. It is usually accompanied by high FSH levels.
Ovarian drilling, also known as multiperforation or laparoscopic ovarian diathermy, is a surgical technique of puncturing the membranes surrounding the ovary with a laser beam or a surgical needle using minimally invasive laparoscopic procedures. It differs from ovarian wedge resection, which involves the cutting of tissue. Minimally invasive ovarian drilling procedures have replaced wedge resections. Ovarian drilling is preferred to wedge resection because cutting into the ovary can cause adhesions which may complicate postoperative outcomes. Ovarian drilling and ovarian wedge resection are treatment options to reduce the amount of androgen producing tissue in women with polycystic ovarian syndrome (PCOS). PCOS is the primary cause of anovulation, which results in female infertility. The induction of mono-ovulatory cycles can restore fertility.

Fertility testing is the process by which fertility is assessed, both generally and also to find the "fertile window" in the menstrual cycle. General health affects fertility, and STI testing is an important related field.
Induction of final maturation of oocytes is a procedure that is usually performed as part of controlled ovarian hyperstimulation to render the oocytes fully developed and thereby resulting in optimal pregnancy chances. It is basically a replacement for the luteinizing hormone (LH) surge whose effects include final maturation in natural menstrual cycles.
Obesity is defined as an abnormal accumulation of body fat, usually 20% or more over an individual's ideal body weight. This is often described as a body mass index (BMI) over 30. However, BMI does not account for whether the excess weight is fat or muscle, and is not a measure of body composition. For most people, however, BMI is an indication used worldwide to estimate nutritional status. Obesity is usually the result of consuming more calories than the body needs and not expending that energy by doing exercise. There are genetic causes and hormonal disorders that cause people to gain significant amounts of weight but this is rare. People in the obese category are much more likely to suffer from fertility problems than people of normal healthy weight.
Gonadotropin surge-attenuating factor (GnSAF) is a nonsteroidal ovarian hormone produced by the granulosa cells of small antral ovarian follicles in females. GnSAF is involved in regulating the secretion of luteinizing hormone (LH) from the anterior pituitary and the ovarian cycle. During the early to mid-follicular phase of the ovarian cycle, GnSAF acts on the anterior pituitary to attenuate LH release, limiting the secretion of LH to only basal levels. At the transition between follicular and luteal phase, GnSAF bioactivity declines sufficiently to permit LH secretion above basal levels, resulting in the mid-cycle LH surge that initiates ovulation. In normally ovulating women, the LH surge only occurs when the oocyte is mature and ready for extrusion. GnSAF bioactivity is responsible for the synchronised, biphasic nature of LH secretion.
References
- ↑ Goldenberg N, Glueck C (2008). "Medical therapy in women with polycystic ovary syndrome before and during pregnancy and lactation". Minerva Ginecol. 60 (1): 63–75. PMID 18277353.
- ↑ Boomsma CM, Fauser BC, Macklon NS (2008). "Pregnancy complications in women with polycystic ovary syndrome". Semin. Reprod. Med. 26 (1): 72–84. doi:10.1055/s-2007-992927. PMID 18181085. S2CID 13930098.
- ↑ Palacio JR, Iborra A, Ulcova-Gallova Z, Badia R, Martínez P (May 2006). "The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients". Clin. Exp. Immunol. 144 (2): 217–22. doi:10.1111/j.1365-2249.2006.03061.x. PMC 1809652 . PMID 16634794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO (June 2004). "The prevalence and features of the polycystic ovary syndrome in an unselected population". J. Clin. Endocrinol. Metab. 89 (6): 2745–9. doi:10.1210/jc.2003-032046. PMID 15181052.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 Gorry, A.; White, D. M.; Franks, S. (August 2006). "Infertility in polycystic ovary syndrome: focus on low-dose gonadotropin treatment". Endocrine . 30 (1): 27–33. doi:10.1385/ENDO:30:1:27. PMID 17185789. S2CID 20751100.
- 1 2 3 4 Brothers, K. J.; Wu, S.; Divall, S. A.; Messmer, M. R.; Kahn, C. R.; Miller, R. S.; Radovick, S.; Wondisford, F. E.; Wolfe, A. (2010). "Rescue of Obesity-Induced Infertility in Female Mice due to a Pituitary-Specific Knockout of the Insulin Receptor (IR)". Cell Metabolism. 12 (3): 295–305. doi:10.1016/j.cmet.2010.06.010. PMC 2935812 . PMID 20816095.
- ↑ Deepak A. Rao; Le, Tao; Bhushan, Vikas (2007). First Aid for the USMLE Step 1 2008 (First Aid for the Usmle Step 1) . McGraw-Hill Medical. ISBN 978-0-07-149868-5.
- 1 2 Qiao, J.; Feng, H. L. (2010). "Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence". Human Reproduction Update. 17 (1): 17–33. doi:10.1093/humupd/dmq032. PMC 3001338 . PMID 20639519.
- ↑ Cunha, Anita; Póvoa, Ana Margarida (26 January 2021). "Infertility management in women with polycystic ovary syndrome: a review". Porto Biomedical Journal. 6 (1): e116. doi:10.1097/j.pbj.0000000000000116. PMC 7846416 . PMID 33532657.
- ↑ "Basal Body Temperature". Pacific Fertility Center. Retrieved 6 March 2015.
- ↑ Benham, J. L.; Yamamoto, J. M.; Friedenreich, C. M.; Rabi, D. M.; Sigal, R. J. (August 2018). "Role of exercise training in polycystic ovary syndrome: a systematic review and meta-analysis". Clinical Obesity. 8 (4): 275–284. doi:10.1111/cob.12258. ISSN 1758-8111. PMID 29896935. S2CID 48355953.
- ↑ Williams, Tracy; Mortada, Rami; Porter, Samuel (2016-07-15). "Diagnosis and Treatment of Polycystic Ovary Syndrome". American Family Physician. 94 (2): 106–13. ISSN 0002-838X. PMID 27419327.
- 1 2 Franik, Sebastian; Le, Quang-Khoi; Kremer, Jan Am; Kiesel, Ludwig; Farquhar, Cindy (2022-09-27). "Aromatase inhibitors (letrozole) for ovulation induction in infertile women with polycystic ovary syndrome". The Cochrane Database of Systematic Reviews. 2022 (9): CD010287. doi:10.1002/14651858.CD010287.pub4. ISSN 1469-493X. PMC 9514207. PMID 36165742.
- 1 2 3 4 Baird, D. T.; Balen, A.; Escobar-Morreale, H. F.; Evers, J. L. H.; Fauser, B. C. J. M.; Franks, S.; Glasier, A.; Homburg, R.; La Vecchia, C.; Devroey, P.; Diedrich, K.; Fraser, L.; Gianaroli, L.; Liebaers, I.; Sunde, A.; Tapanainen, J. S.; Tarlatzis, B.; Van Steirteghem, A.; Veiga, A.; Crosignani, P. G.; Evers, J. L. H. (2012). "Health and fertility in World Health Organization group 2 anovulatory women". Human Reproduction Update. 18 (5): 586–599. doi:10.1093/humupd/dms019. PMID 22611175.
- 1 2 3 Misso, M. L.; Costello, M. F.; Garrubba, M.; Wong, J.; Hart, R.; Rombauts, L.; Melder, A. M.; Norman, R. J.; Teede, H. J. (2012). "Metformin versus clomiphene citrate for infertility in non-obese women with polycystic ovary syndrome: A systematic review and meta-analysis". Human Reproduction Update. 19 (1): 2–11. doi: 10.1093/humupd/dms036 . PMID 22956412.
- 1 2 Tang, T.; Balen, A. H. (2012). "Use of metformin for women with polycystic ovary syndrome". Human Reproduction Update. 19 (1): 1. doi: 10.1093/humupd/dms040 . PMID 23114640.
- ↑ Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (March 2008). "Consensus on infertility treatment related to polycystic ovary syndrome". Fertil. Steril. 89 (3): 505–22. doi:10.1016/j.fertnstert.2007.09.041. PMID 18243179.
- ↑ Johnson NP, Stewart AW, Falkiner J, et al. (April 2010). "PCOSMIC: a multi-centre randomized trial in women with PolyCystic Ovary Syndrome evaluating Metformin for Infertility with Clomiphene". Hum Reprod. 25 (7): 1675–83. doi:10.1093/humrep/deq100. PMID 20435692.
- ↑ Farquhar, Cindy; Marjoribanks, Jane (17 August 2018). "Assisted reproductive technology: an overview of Cochrane Reviews". The Cochrane Database of Systematic Reviews. 2018 (8): CD010537. doi:10.1002/14651858.CD010537.pub5. ISSN 1469-493X. PMC 6953328 . PMID 30117155.