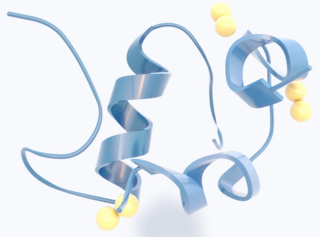
Insulin is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (INS) gene. It is the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats, and protein by promoting the absorption of glucose from the blood into cells of the liver, fat, and skeletal muscles. In these tissues the absorbed glucose is converted into either glycogen, via glycogenesis, or fats (triglycerides), via lipogenesis; in the liver, glucose is converted into both. Glucose production and secretion by the liver are strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is thus an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules in the cells. Low insulin in the blood has the opposite effect, promoting widespread catabolism, especially of reserve body fat.
Intensive insulin therapy or flexible insulin therapy is a therapeutic regimen for diabetes mellitus treatment. This newer approach contrasts with conventional insulin therapy. Rather than minimize the number of insulin injections per day, the intensive approach favors flexible meal times with variable carbohydrate as well as flexible physical activities. The trade-off is the increase from 2 or 3 injections per day to 4 or more injections per day, which was considered "intensive" relative to the older approach. In North America in 2004, many endocrinologists prefer the term "flexible insulin therapy" (FIT) to "intensive therapy" and use it to refer to any method of replacing insulin that attempts to mimic the pattern of small continuous basal insulin secretion of a working pancreas combined with larger insulin secretions at mealtimes. The semantic distinction reflects changing treatment.
Drugs used in diabetes treat diabetes mellitus by decreasing glucose levels in the blood. With the exception of insulin, most GLP-1 receptor agonists, and pramlintide, all diabetes medications are administered orally and are thus called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of hypoglycemic drugs, and selection of the appropriate agent depends on the nature of diabetes, age, and situation of the person, as well as other patient factors.
Feline diabetes mellitus is a chronic disease in cats whereby either insufficient insulin response or insulin resistance leads to persistently high blood glucose concentrations. Diabetes affects up to 1 in 230 cats, and may be becoming increasingly common. Diabetes is less common in cats than in dogs. The condition is treatable, and if treated properly the cat can experience a normal life expectancy. In cats with type 2 diabetes, prompt effective treatment may lead to diabetic remission, in which the cat no longer needs injected insulin. Untreated, the condition leads to increasingly weak legs in cats and eventually to malnutrition, ketoacidosis and/or dehydration, and death.

Insulin glargine sold under the brand name Lantus among others is a long-acting modified form of medical insulin, used in the management of type 1 and type 2 diabetes. It is injected just under the skin. Effects generally begin an hour after use.
Neutral Protamine Hagedorn (NPH) insulin, also known as isophane insulin, is an intermediate-acting insulin given to help control blood sugar levels in people with diabetes. The words refer to neutral pH, protamine a protein, and Hans Christian Hagedorn, the insulin researcher who invented this formulation. It is designed to improve the delivery of insulin, and is one of the earliest examples of engineered drug delivery.

Insulin glulisine, sold under the brand name Apidra among others, is a rapid-acting modified form of medical insulin used for the treatment of diabetes. It differs from human insulin in that the amino acid asparagine at position B3 is replaced by lysine and the lysine in position B29 is replaced by glutamic acid. When injected subcutaneously, it appears in the blood earlier than regular human insulin (RHI). Intravenous injections may be used for extreme hyperglycemia. It was developed by Sanofi-Aventis.
Insulin detemir, sold under the brand name Levemir among others, is a long-acting modified form of medical insulin used to treat both type 1 and type 2 diabetes. It is used by injection under the skin. It is effective for up to 24 hours.

Insulin aspart, sold under the brand name NovoLog, among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is generally used by injection under the skin but may also be used by injection into a vein. Maximum effect occurs after about 1–3 hours and lasts for 3–5 hours. Generally a longer-acting insulin like insulin NPH is also needed.

Insulin lispro, sold under the brand name Humalog among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is delivered subcutaneously either by injection or from an insulin pump. Onset of effects typically occurs within 30 minutes and lasts about 5 hours. Often a longer-acting insulin like insulin NPH is also needed.

Liraglutide, sold under the brand name Victoza among others, is an anti-diabetic medication used to treat type 2 diabetes, and chronic obesity. It is a second-line therapy for diabetes following first-line therapy with metformin. Its effects on long-term health outcomes like heart disease and life expectancy are unclear. It is given by injection under the skin.

Regular insulin, also known as neutral insulin and soluble insulin, is a type of short-acting medical insulin. It is used to treat type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. It is also used along with glucose to treat high blood potassium levels. Typically it is given by injection under the skin, but may also be used by injection into a vein or muscle. Onset of effect is typically in 30 minutes and it typically lasts for 8 hours.
Albiglutide is a glucagon-like peptide-1 agonist drug marketed by GlaxoSmithKline (GSK) for treatment of type 2 diabetes. As of 2017 it is unclear if it affects a person's risk of death. In 2017 GSK announced Albiglutide's withdrawal from the worldwide market for economic reasons, and remaining stocks in the supply chain were effectively depleted by 2018.
As a medication, insulin is any pharmaceutical preparation of the protein hormone insulin that is used to treat high blood glucose. Such conditions include type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. Insulin is also used along with glucose to treat hyperkalemia. Typically it is given by injection under the skin, but some forms may also be used by injection into a vein or muscle. There are various types of insulin, suitable for various time spans. The types are often all called insulin in the broad sense, although in a more precise sense, insulin is identical to the naturally occurring molecule whereas insulin analogues have slightly different molecules that allow for modified time of action. It is on the World Health Organization's List of Essential Medicines. In 2022, it was the 192nd most commonly prescribed medication in the United States, with more than 2 million prescriptions.

Lente insulin was an intermediate duration insulin that is no longer used in humans. The onset of lente insulin is one to two hours after the dose is administered, and the peak effect is approximately 8 to 12 hours after administration, with some effects lasting over 24 hours.

Insulin degludec (INN/USAN) is an ultralong-acting basal insulin analogue that was developed by Novo Nordisk under the brand name Tresiba. It is administered via subcutaneous injection to help control the blood sugar level of those with diabetes. It has a duration of action that lasts up to 42 hours, making it a once-daily basal insulin, that is one that provides a base insulin level, as opposed to the fast- and short-acting bolus insulins.
Ultralente insulin was a long-acting form of insulin. It has an onset of 4 to 6 hours, a peak of 14 to 24 hours, and a duration of 28 to 36 hours. Ultralente insulin, along with lente insulin, were discontinued in the US by manufacturers in the mid-2000s. One of the reasons for discontinuation was declining use in favor of NPH insulin and other newer insulin products. The FDA withdrew approval for ultralente insulin products by 2011.
Insulin glargine/lixisenatide, sold under the brand name Soliqua among others, is a fixed-dose combination medication that combines insulin glargine and lixisenatide and is used to treat diabetes.

Tirzepatide is an antidiabetic medication used for the treatment of type 2 diabetes and for weight loss. Tirzepatide is administered via subcutaneous injections. It is sold under the brand name Mounjaro for diabetes treatment, and Zepbound for weight loss and treatment of obstructive sleep apnea.
Insulin icodec, sold under the brand name Awiqli, is a medication used for the treatment of diabetes to improve glycemic control. It is an ultralong-acting basal insulin analogue that is developed by Novo Nordisk.













