Related Research Articles

A hydrogel is a biphasic material, a mixture of porous and permeable solids and at least 10% of water or other interstitial fluid. The solid phase is a water insoluble three dimensional network of polymers, having absorbed a large amount of water or biological fluids. Hydrogels have several applications, especially in the biomedical area, such as in hydrogel dressing. Many hydrogels are synthetic, but some are derived from natural materials. The term "hydrogel" was coined in 1894.
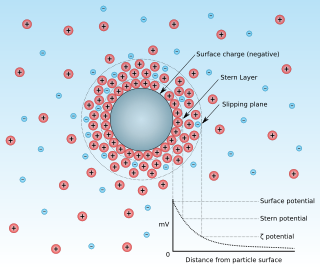
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.

Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describe their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectrolytes. Both natural and synthetic polyelectrolytes are used in a variety of industries.
Microencapsulation is a process in which tiny particles or droplets are surrounded by a coating to give small capsules, with useful properties. In general, it is used to incorporate food ingredients, enzymes, cells or other materials on a micro metric scale. Microencapsulation can also be used to enclose solids, liquids, or gases inside a micrometric wall made of hard or soft soluble film, in order to reduce dosing frequency and prevent the degradation of pharmaceuticals.

An iodophor is a preparation containing iodine complexed with a solubilizing agent, such as a surfactant or water-soluble polymers such as povidone, The result is a water-soluble material that releases free iodine when in solution.

Coacervate is an aqueous phase rich in macromolecules such as synthetic polymers, proteins or nucleic acids. It forms through liquid-liquid phase separation (LLPS), leading to a dense phase in thermodynamic equilibrium with a dilute phase. The dispersed droplets of dense phase are also called coacervates, micro-coacervates or coacervate droplets. These structures draw a lot of interest because they form spontaneously from aqueous mixtures and provide stable compartmentalization without the need of a membrane—they are protocell candidates.
A dispersion is a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene flanked by two hydrophilic chains of polyoxyethylene. The word poloxamer was coined by BASF inventor, Irving Schmolka, who received the patent for these materials in 1973. Poloxamers are also known by the trade names Pluronic, Kolliphor, and Synperonic.
Small-angle X-ray scattering (SAXS) is a small-angle scattering technique by which nanoscale density differences in a sample can be quantified. This means that it can determine nanoparticle size distributions, resolve the size and shape of (monodisperse) macromolecules, determine pore sizes, characteristic distances of partially ordered materials, and much more. This is achieved by analyzing the elastic scattering behaviour of X-rays when travelling through the material, recording their scattering at small angles. It belongs to the family of small-angle scattering (SAS) techniques along with small-angle neutron scattering, and is typically done using hard X-rays with a wavelength of 0.07 – 0.2 nm. Depending on the angular range in which a clear scattering signal can be recorded, SAXS is capable of delivering structural information of dimensions between 1 and 100 nm, and of repeat distances in partially ordered systems of up to 150 nm. USAXS can resolve even larger dimensions, as the smaller the recorded angle, the larger the object dimensions that are probed.
Layer-by-layer (LbL) deposition is a thin film fabrication technique. The films are formed by depositing alternating layers of complementary materials with wash steps in between. This can be accomplished by using various techniques such as immersion, spin, spray, electromagnetism, or fluidics.
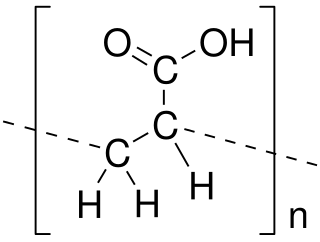
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2−CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof, are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid–base and water-attracting – are the bases of many applications.
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis. Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms. Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.
Adsorption of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.
Polyelectrolytes are charged polymers capable of stabilizing colloidal emulsions through electrostatic interactions. Their effectiveness can be dependent on molecular weight, pH, solvent polarity, ionic strength, and the hydrophilic-lipophilic balance (HLB). Stabilized emulsions are useful in many industrial processes, including deflocculation, drug delivery, petroleum waste treatment, and food technology.

Interfacial polymerization is a type of step-growth polymerization in which polymerization occurs at the interface between two immiscible phases, resulting in a polymer that is constrained to the interface. There are several variations of interfacial polymerization, which result in several types of polymer topologies, such as ultra-thin films, nanocapsules, and nanofibers, to name just a few.

Andreas Bernkop-Schnürch is an Austrian scientist and entrepreneur, who is Head of the Department of Pharmaceutical Technology in the Institute of Pharmacy at the University of Innsbruck.
Chitosan-poly is a composite that has been increasingly used to create chitosan-poly(acrylic acid) nanoparticles. More recently, various composite forms have come out with poly(acrylic acid) being synthesized with chitosan which is often used in a variety of drug delivery processes. Chitosan which already features strong biodegradability and biocompatibility nature can be merged with polyacrylic acid to create hybrid nanoparticles that allow for greater adhesion qualities as well as promote the biocompatibility and homeostasis nature of chitosan poly(acrylic acid) complex. The synthesis of this material is essential in various applications and can allow for the creation of nanoparticles to facilitate a variety of dispersal and release behaviors and its ability to encapsulate a multitude of various drugs and particles.
Vitaliy Khutoryanskiy FRSC FAPS is a British and Kazakhstani scientist, a Professor of Formulation Science and a Royal Society Industry Fellow at the University of Reading. His research focuses on polymers, biomaterials, nanomaterials, drug delivery, and pharmaceutical sciences. Khutoryanskiy has published over 200 original research articles, book chapters, and reviews. His publications have attracted > 12000 citations and his current h-index is 54. He received several prestigious awards in recognition for his research in polymers, colloids and drug delivery as well as for contributions to research peer-review and mentoring of early career researchers. He holds several honorary professorship titles from different universities.

Valentin Alekseyevich Kargin was a Soviet and Russian chemist who specialized in physical chemistry and established research in polymer chemistry in the Soviet Union. He considered polymerization as a phase transition.

Alexander Viktorovich Kabanov, is a Russian and American chemist, an educator, an entrepreneur, and a researcher in the fields of drug delivery and nanomedicine.
References
- ↑ Tsuchida, E.; Abe, K., eds. (1982). "Interactions Between Macromolecules in Solution and Intermacromolecular Complexes". Advances in Polymer Science. 45. doi:10.1007/bfb0017548. ISBN 3-540-11624-9.
- Bekturov, Esen A.; Bimendina, Larisa A. (1981). "Interpolymer complexes". Speciality Polymers. Advances in Polymer Science. 41. Springer Berlin Heidelberg: 99–147. doi:10.1007/3-540-10554-9_11. ISBN 978-3-540-38525-7. - ↑ Kabanov, V. (2002), "Fundamentals of Polyelectrolyte Complexes in Solution and the Bulk", Multilayer Thin Films, Wiley-VCH Verlag GmbH & Co. KGaA, pp. 47–86, doi:10.1002/3527600574.ch2, ISBN 3-527-30440-1
- ↑ Khutoryanskiy, Vitaliy V.; Staikos, Georgios (2009). Hydrogen-bonded interpolymer complexes : formation, structure and applications. Singapore: World Scientific Pub. Co. ISBN 978-981-270-977-6. OCLC 613658891.
- ↑ Tsuji, Hideto (December 2016). "Poly(lactic acid) stereocomplexes: A decade of progress". Advanced Drug Delivery Reviews. 107: 97–135. doi:10.1016/j.addr.2016.04.017. PMID 27125192.
- ↑ Peredereeva, S I; Orlov, I G; Cherkashin, Mikhail I (1975-04-30). "Polymeric Charge-transfer Complexes". Russian Chemical Reviews. 44 (4): 295–305. Bibcode:1975RuCRv..44..295P. doi:10.1070/rc1975v044n04abeh002268. ISSN 0036-021X.
- ↑ Murmiliuk, Anastasiia; Hladysh, Sviatoslav; Filippov, Sergey K.; Stepanek, Miroslav (2022-09-01). "Comprehensive Multidimensional Characterization of Polyelectrolytes and Interpolyelectrolyte Complexes in Aqueous Solutions". Reviews and Advances in Chemistry. 12 (3): 163–177. doi:10.1134/S263482762260013X. ISSN 2634-8284.
- ↑ Khutoryanskiy, V. V.; Smyslov, R. Yu.; Yakimansky, A. V. (2018-09-01). "Modern Methods for Studying Polymer Complexes in Aqueous and Organic Solutions" (PDF). Polymer Science, Series A. 60 (5): 553–576. doi:10.1134/S0965545X18050085. ISSN 1555-6107.
- ↑ Khutoryanskiy, Vitaliy V. (2007-04-04). "Hydrogen-bonded interpolymer complexes as materials for pharmaceutical applications". International Journal of Pharmaceutics. 334 (1): 15–26. doi:10.1016/j.ijpharm.2007.01.037. ISSN 0378-5173. PMID 17320317.
- ↑ Fay, James M.; Kabanov, Alexander V. (2022-09-01). "Interpolyelectrolyte Complexes as an Emerging Technology for Pharmaceutical Delivery of Polypeptides". Reviews and Advances in Chemistry. 12 (3): 137–162. doi:10.1134/S2634827622600177. ISSN 2634-8284. PMC 9987408 .
- ↑ Murmiliuk, Anastasiia; Iwase, Hiroki; Kang, Jia-Jhen; Mohanakumar, Shilpa; Appavou, Marie-Sousai; Wood, Kathleen; Almásy, László; Len, Adél; Schwärzer, Kuno; Allgaier, Jürgen; Dulle, Martin; Gensch, Thomas; Förster, Beate; Ito, Kanae; Nakagawa, Hiroshi (2024). "Polyelectrolyte-protein synergism: pH-responsive polyelectrolyte/insulin complexes as versatile carriers for targeted protein and drug delivery". Journal of Colloid and Interface Science. 665: 801–813. doi:10.1016/j.jcis.2024.03.156. ISSN 0021-9797.
- ↑ Decher, Gero (1997-08-29). "Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites". Science. 277 (5330): 1232–1237. doi:10.1126/science.277.5330.1232. ISSN 0036-8075.
- ↑ Bromberg, L. E. (1991-10-01). "Composite membranes based on polyelectrolyte complexes". Journal of Membrane Science. 62 (2): 131–143. doi:10.1016/0376-7388(91)80057-D. ISSN 0376-7388.
- ↑ Zezin, A. B.; Mikheikin, S. V.; Rogacheva, V. B.; Zansokhova, M. F.; Sybachin, A. V.; Yaroslavov, A. A. (2015-12-01). "Polymeric stabilizers for protection of soil and ground against wind and water erosion". Advances in Colloid and Interface Science. Colloid and Polymer Interfaces in Bio-resources and Environments. 226 (Pt A): 17–23. doi:10.1016/j.cis.2015.06.006. ISSN 0001-8686. PMID 26260276.
- ↑ Geest, Bruno G. De; Koker, Stefaan De; Sukhorukov, Gleb B.; Kreft, Oliver; Parak, Wolfgang J.; Skirtach, Andrei G.; Demeester, Jo; Smedt, Stefaan C. De; Hennink, Wim E. (2009-01-08). "Polyelectrolyte microcapsules for biomedical applications". Soft Matter. 5 (2): 282–291. Bibcode:2009SMat....5..282D. doi:10.1039/B808262F. ISSN 1744-6848.