Genetics
| | This section is empty. You can help by adding to it. (October 2021) |
| Isolated hypogonadotropic hypogonadism | |
|---|---|
| Other names | Normosmic idiopathic hypogonadotropic hypogonadism |
Isolated hypogonadotropic hypogonadism (IHH), also called idiopathic or congenital hypogonadotropic hypogonadism (CHH), as well as isolated or congenital gonadotropin-releasing hormone deficiency (IGD), is a condition which results in a small subset of cases of hypogonadotropic hypogonadism (HH) due to deficiency in or insensitivity to gonadotropin-releasing hormone (GnRH) where the function and anatomy of the anterior pituitary is otherwise normal and secondary causes of HH are not present.[ citation needed ]
Congenital hypogonadotropic hypogonadism presents as hypogonadism, e.g., reduced or absent puberty, [1] low libido, infertility, etc. due to an impaired release of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and a resultant lack of sex steroid and peptides production by the gonads. [2] [3]
In Kallmann syndrome, a variable non-reproductive phenotype occurs with anosmia (loss of the sense of smell) including sensorineural deafness, coloboma, bimanual synkinesis, craniofacial abnormalities, and/or renal agenesis. [4]
IHH is divided into two syndromes: IHH with olfactory alterations or anosmia, Kallmann syndrome and IHH with normal smell (normosmic IHH). [4]
Kallmann syndrome is responsible for approximately 50% of all cases of the condition. It is associated with mutations in KAL1 , FGFR1/FGF8 , FGF17 , IL17RD , PROKR2 , NELF , CHD7 (which positively regulates GnRH secretion), HS6ST1, FLRT3 , SPRY4 , DUSP6, SEMA3A , and WDR11 (gene) , genes which are related to defects in neuronal migration. [4]
Gene defects associated with IHH and normal smell include PROKR2, FGFR1, FGF8, CHD7, DUSP6, and WDR11, as in KS, but in addition mutations in KISS1R , TACR3 , GNRH1/GNRHR, LEP/LEPR, HESX1, FSHB, and LHB. [4] GnRH insensitivity is the second most common cause of IHH, responsible for up to 20% of cases.[ citation needed ]A minority of less than 5-10% is due to inactivating mutations in genes which positively regulate GnRH secretion such as CHD7, KISS1R, and TACR3.[ citation needed ]
The causes of about 25% of all IHH cases are still unknown. [5]
| | This section is empty. You can help by adding to it. (October 2021) |
| | This section is empty. You can help by adding to it. (November 2017) |

Follicle-stimulating hormone (FSH) is a gonadotropin, a glycoprotein polypeptide hormone. FSH is synthesized and secreted by the gonadotropic cells of the anterior pituitary gland and regulates the development, growth, pubertal maturation, and reproductive processes of the body. FSH and luteinizing hormone (LH) work together in the reproductive system.
Anovulation is when the ovaries do not release an oocyte during a menstrual cycle. Therefore, ovulation does not take place. However, a woman who does not ovulate at each menstrual cycle is not necessarily going through menopause. Chronic anovulation is a common cause of infertility.
Hypogonadism means diminished functional activity of the gonads—the testes or the ovaries—that may result in diminished production of sex hormones. Low androgen levels are referred to as hypoandrogenism and low estrogen as hypoestrogenism. These are responsible for the observed signs and symptoms in both males and females.
Kallmann syndrome (KS) is a genetic disorder that prevents a person from starting or fully completing puberty. Kallmann syndrome is a form of a group of conditions termed hypogonadotropic hypogonadism. To distinguish it from other forms of hypogonadotropic hypogonadism, Kallmann syndrome has the additional symptom of a total lack of sense of smell (anosmia) or a reduced sense of smell. If left untreated, people will have poorly defined secondary sexual characteristics, show signs of hypogonadism, almost invariably are infertile and are at increased risk of developing osteoporosis. A range of other physical symptoms affecting the face, hands and skeletal system can also occur.
X-linked adrenal hypoplasia congenita is a genetic disorder that mainly affects males. It involves many endocrine tissues in the body, especially the adrenal glands.
The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone releasing hormone receptor (LHRHR), is a member of the seven-transmembrane, G-protein coupled receptor (GPCR) family. It is the receptor of gonadotropin-releasing hormone (GnRH). The GnRHR is expressed on the surface of pituitary gonadotrope cells as well as lymphocytes, breast, ovary, and prostate.
Anosmin-1 is a secreted, EM associated glycoprotein found in humans and other organisms responsible for normal development, which is expressed in the brain, spinal cord and kidney. Absence or damage to the protein results in Kallmann syndrome in humans, which is characterized by loss of olfactory bulbs and GnRH secretion leading to anosmia and hypothalamic hypogonadotropic hypogonadism. Anosmin-1 is coded by the KAL-1 gene, which is found on the X chromosome. Anosmin-1 is 100 kilodaltons and is expressed on the outside of cells. Because of this and because of its contribution to normal migration of nerve cells, a role in the extracellular matrix has been postulated.

Gonadotropin-releasing hormone receptor is a protein that in humans is encoded by the GNRHR gene.

Homeobox protein prophet of PIT-1 is a protein that in humans is encoded by the PROP1 gene.
Progonadoliberin-2 is a protein that in humans is encoded by the GNRH2 gene.

Luteinizing hormone subunit beta also known as lutropin subunit beta or LHβ is a polypeptide that in association with an alpha subunit common to all gonadotropin hormones forms the reproductive signaling molecule luteinizing hormone. In humans it is encoded by the LHB gene.

Aromatase deficiency is a rare condition characterized by extremely low levels or complete absence of the enzyme aromatase activity in the body. It is an autosomal recessive disease resulting from various mutations of gene CYP19 (P450arom) which can lead to ambiguous genitalia and delayed puberty in females, continued linear growth into adulthood and osteoporosis in males and virilization in pregnant mothers. As of 2020, fewer than 15 cases have been identified in genetically male individuals and at least 30 cases in genetically female individuals.

Isolated 17,20-lyase deficiency (ILD), also called isolated 17,20-desmolase deficiency, is a rare endocrine and autosomal recessive genetic disorder which is characterized by a complete or partial loss of 17,20-lyase activity and, in turn, impaired production of the androgen and estrogen sex steroids. The condition manifests itself as pseudohermaphroditism in males, in whom it is considered to be a form of intersex, and, in both sexes, as a reduced or absent puberty/lack of development of secondary sexual characteristics, resulting in a somewhat childlike appearance in adulthood.
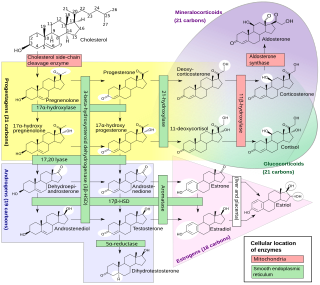
An inborn error of steroid metabolism is an inborn error of metabolism due to defects in steroid metabolism.
Gonadotropin-releasing hormone (GnRH) insensitivity also known as Isolated gonadotropin-releasing hormone (GnRH)deficiency (IGD) is a rare autosomal recessive genetic and endocrine syndrome which is characterized by inactivating mutations of the gonadotropin-releasing hormone receptor (GnRHR) and thus an insensitivity of the receptor to gonadotropin-releasing hormone (GnRH), resulting in a partial or complete loss of the ability of the gonads to synthesize the sex hormones. The condition manifests itself as isolated hypogonadotropic hypogonadism (IHH), presenting with symptoms such as delayed, reduced, or absent puberty, low or complete lack of libido, and infertility, and is the predominant cause of IHH when it does not present alongside anosmia.
Hypogonadotropic hypogonadism (HH), is due to problems with either the hypothalamus or pituitary gland affecting the hypothalamic-pituitary-gonadal axis. Hypothalamic disorders result from a deficiency in the release of gonadotropic releasing hormone (GnRH), while pituitary gland disorders are due to a deficiency in the release of gonadotropins from the anterior pituitary. GnRH is the central regulator in reproductive function and sexual development via the HPG axis. GnRH is released by GnRH neurons, which are hypothalamic neuroendocrine cells, into the hypophyseal portal system acting on gonadotrophs in the anterior pituitary. The release of gonadotropins, LH and FSH, act on the gonads for the development and maintenance of proper adult reproductive physiology. LH acts on Leydig cells in the male testes and theca cells in the female. FSH acts on Sertoli cells in the male and follicular cells in the female. Combined this causes the secretion of gonadal sex steroids and the initiation of folliculogenesis and spermatogenesis. The production of sex steroids forms a negative feedback loop acting on both the anterior pituitary and hypothalamus causing a pulsatile secretion of GnRH. GnRH neurons lack sex steroid receptors and mediators such as kisspeptin stimulate GnRH neurons for pulsatile secretion of GnRH.

GnRH neurons, or gonadotropin-releasing hormone expressing neurons, are the cells in the brain that control the release of reproductive hormones from the pituitary. These brain cells control reproduction by secreting GnRH into the hypophyseal portal capillary bloodstream, so are sometimes referred to as “sex neurons”. This small capillary network carries GnRH to the anterior pituitary, causing release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) into the wider bloodstream. When GnRH neurons change their pattern of release from the juvenile to the adult pattern of GnRH secretion, puberty is initiated. Failure of GnRH neurons to form the proper connections, or failure to successfully stimulate the pituitary with GnRH, means that puberty is not initiated. These disruptions to the GnRH system cause reproductive disorders like hypogonadotropic hypogonadism or Kallmann Syndrome.

The fertile eunuch syndrome or Pasqualini syndrome is a cause of hypogonadotropic hypogonadism caused by a luteinizing hormone deficiency. It is characterized by hypogonadism with spermatogenesis. Pasqualini and Bur published the first case of eunuchoidism with preserved spermatogenesis in 1950 in la Revista de la Asociación Médica Argentina. The hypoandrogenism with spermatogenesis syndrome included:

To date, at least 25 different genes have been implicated in causing gonadotropin-releasing hormone (GnRH) deficiency conditions such as Kallmann syndrome (KS) or other forms of congenital hypogonadotropic hypogonadism (CHH) through a disruption in the production or activity of GnRH. These genes involved cover all forms of inheritance, and no one gene defect has been shown to be common to all cases, which makes genetic testing and inheritance prediction difficult.

Anosmin 1 is a protein that in humans is encoded by the ANOS1 gene.