Related Research Articles
An electrolyte is a medium containing ions that is electrically conducting through the movement of ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.

A lithium polymer battery, or more correctly lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. High conductivity semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, such as mobile devices, radio-controlled aircraft and some electric vehicles.

Aviation fuels are petroleum-based fuels, or petroleum and synthetic fuel blends, used to power aircraft. They have more stringent requirements than fuels used for ground use, such as heating and road transport, and contain additives to enhance or maintain properties important to fuel performance or handling. They are kerosene-based for gas turbine-powered aircraft. Piston-engined aircraft use gasoline and those with diesel engines may use jet fuel (kerosene). By 2012 all aircraft operated by the U.S. Air Force had been certified to use a 50-50 blend of kerosene and synthetic fuel derived from coal or natural gas as a way of stabilizing the cost of fuel.

Jet fuel or aviation turbine fuel is a type of aviation fuel designed for use in aircraft powered by gas-turbine engines. It is colorless to straw-colored in appearance. The most commonly used fuels for commercial aviation are Jet A and Jet A-1, which are produced to a standardized international specification. The only other jet fuel commonly used in civilian turbine-engine powered aviation is Jet B, which is used for its enhanced cold-weather performance.

Potassium peroxymonosulfate is widely used as an oxidizing agent. It is the potassium salt of peroxymonosulfuric acid. Usually potassium peroxymonosulfate refers to the triple salt known as oxone.
JP-4, or JP4 was a jet fuel, specified in 1951 by the U.S. government (MIL-DTL-5624). Its NATO code is F-40. It is also known as avtag.

A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion exchange occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts.
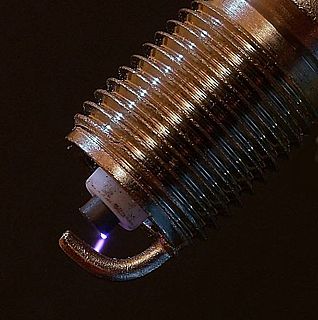
An electric spark is an abrupt electrical discharge that occurs when a sufficiently high electric field creates an ionized, electrically conductive channel through a normally-insulating medium, often air or other gases or gas mixtures. Michael Faraday described this phenomenon as "the beautiful flash of light attending the discharge of common electricity".

An aviation biofuel or bio-jet-fuel or bio-aviation fuel (BAF) is a biofuel used to power aircraft and is said to be a sustainable aviation fuel (SAF). The International Air Transport Association (IATA) considers it a key element to reducing the carbon footprint within the environmental impact of aviation. Aviation biofuel could help decarbonize medium- and long-haul air travel generating most emissions, and could extend the life of older aircraft types by lowering their carbon footprint.
Photoelectrochemical reduction of carbon dioxide is a chemical process whereby carbon dioxide is reduced to carbon monoxide or hydrocarbons by the energy of incident light. This process requires catalysts, most of which are semiconducting materials. The feasibility of this chemical reaction was first theorised by Giacomo Luigi Ciamician, an Italian photochemist. Already in 1912 he stated that "[b]y using suitable catalyzers, it should be possible to transform the mixture of water and carbon dioxide into oxygen and methane, or to cause other endo-energetic processes."

Electrofuels or e-fuels are an emerging class of drop-in replacement fuels that are made by storing energy from renewable sources in the chemical bonds of liquid or gas fuels, aiming to be a carbon-neutral fuel. They are an alternative to aviation biofuel. The primary targets are butanol, biodiesel, and hydrogen, but include other alcohols and carbon-containing gases such as methane and butane.
Methylpyridinium is a chemical compound which is the quaternary ammonium compound derived from the N-methylation of pyridine. It is found in some coffee products. It is not present in unroasted coffee beans, but is formed during roasting from its precursor chemical, trigonelline. It is under investigation by scientists regarding its potential anti-carcinogenic properties, particularly an effect on colon cancer.
Worldwide commercial synthetic fuels plant capacity is over 240,000 barrels per day (38,000 m3/d), including indirect conversion Fischer–Tropsch plants in South Africa, Qatar, and Malaysia, and a Mobil process plant in New Zealand.

A solid dispersion redox flow battery is a type of redox flow battery using dispersed solid active materials as the energy storage media. The solid suspensions are stored in energy storage tanks and pumped through electrochemical cells while charging or discharging. In comparison with a conventional redox flow battery where active species are dissolved in aqueous or organic electrolyte, the active materials in a solid dispersion redox flow battery maintain the solid form and are suspended in the electrolyte. Further development expanded the applicable active materials. The solid active materials, especially with active materials from lithium-ion battery, can help the suspensions achieve much higher energy densities than conventional redox flow batteries. This concept is similar to semi-solid flow batteries in which slurries of active materials accompanied by conductive carbon additives to facilitate electrons conducting are stored in energy storage tanks and pumped through the electrochemical reaction cells. Based upon this technique, an analytical method was developed to measure the electrochemical performance of lithium-ion battery active materials, named dispersed particle resistance (DPR).
Dispersed particle resistance (DPR) is a measured parameter to characterize battery active materials. It is seen as an indicator of lithium-ion battery active material rate capability. It is the slope of voltage-current linear fit for active material samples in suspensions. It can be obtained by applying different voltages on a suspension and measuring the currents, after which the data points are plotted. The slope of the plot is referred to as dispersed particle resistance. It can also be done in the opposite way where different currents are applied and voltages are measured. The key advantage of this dispersed particle resistance technique is fast and accurate comparing with the conventional characterization method for which batteries need to be fabricated and tested for a long time.

Doron Aurbach is an Israeli electrochemist, materials and surface scientist.
Scanning vibrating electrode technique (SVET), also known as vibrating probe within the field of biology, is a scanning probe microscopy (SPM) technique which visualizes electrochemical processes at a sample. It was originally introduced in 1974 by Jaffe and Nuccitelli to investigate the electrical current densities near living cells. Starting in the 1980s Hugh Isaacs began to apply SVET to a number of different corrosion studies. SVET measures local current density distributions in the solution above the sample of interest, to map electrochemical processes in situ as they occur. It utilizes a probe, vibrating perpendicular to the sample of interest, to enhance the measured signal. It is related to scanning ion-selective electrode technique (SIET), which can be used with SVET in corrosion studies, and scanning reference electrode technique (SRET), which is a precursor to SVET.

Kristina Edström is a Swedish Professor of Inorganic Chemistry at Uppsala University. She also serves as Head of the Ångström Advanced Battery Centre (ÅABC) and has previously been both Vice Dean for Research at the Faculty of Science and Technology and Chair of the STandUp for Energy research programme.

Electrochemical quartz crystal microbalance (EQCM) is the combination of electrochemistry and quartz crystal microbalance, which was generated in the eighties. Typically, an EQCM device contains an electrochemical cells part and a QCM part. Two electrodes on both sides of the quartz crystal serve two purposes. Firstly, an alternating electric field is generated between the two electrodes for making up the oscillator. Secondly, the electrode contacting electrolyte is used as a working electrode (WE), together with a counter electrode (CE) and a reference electrode (RE), in the potentiostatic circuit constituting the electrochemistry cell. Thus, the working electrode of electrochemistry cell is the sensor of QCM.

Debbie Silvester is a British-Australian chemist who is a professor at Curtin University. Her research considers electrochemical processes and sensing. She has explored room-temperature ionic liquids. In 2021, she was awarded the Australian Academy of Science Le Fèvre Medal.
References
- 1 2 3 4 "Networks and Volunteers Working Group". Alumni. Retrieved 31 May 2018.
- ↑ "Splendid Bowling Competition". Tattenhall Online. Retrieved 31 May 2018.
- ↑ Bauldreay, J.M.; Archer, M.D. (1 November 1983). "Dye-modified electrodes for photogalvanic cells". Electrochimica Acta. 28 (11): 1515–1522. doi:10.1016/0013-4686(83)85210-4. ISSN 0013-4686.
- ↑ Bauldreay, J.M.; Archer, M.D. (1 October 1985). "Mediated redox reactions at the 1-aminophenazine-modified rotating disc electrode". Electrochimica Acta. 30 (10): 1355–1359. doi:10.1016/0013-4686(85)85014-3. ISSN 0013-4686.
- ↑ "SOLAR-JET - Partners". www.solar-jet.aero. Retrieved 31 May 2018.
- ↑ "AIAA To Host "Inside Aerospace" - An International Forum For Aerospace Leaders On Energy And Environmental Challenges". www.aerospaceonline.com. Retrieved 31 May 2018.
- ↑ Liquid fuel compositions, 28 April 2010, retrieved 31 May 2018
- ↑ Kerosene base fuel, 17 March 2009, retrieved 31 May 2018
- ↑ Methods of providing higher quality liquid kerosene based-propulsion fuels, 21 December 2016, retrieved 31 May 2018
- ↑ Process for the preparation of middle distillates from kerogen, 17 March 2008, retrieved 31 May 2018
- ↑ Bauldreay, Joanna (8 October 2012). "Alternative Fuels - Alternatives and Production" (PDF). Air Transport Net. Retrieved 31 May 2018.
- ↑ Admin, MemberClicks. "The IASH Lifetime Achievement Award". www.iash.net. Retrieved 31 May 2018.