
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is deoxyribose, a variant of ribose, the polymer is DNA.

Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.
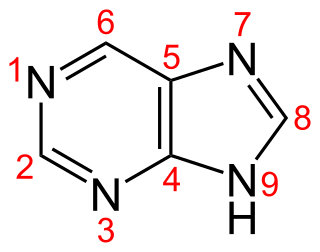
Purine is a heterocyclic aromatic organic compound that consists of two rings fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature.
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

The RNA world is a hypothetical stage in the evolutionary history of life on Earth in which self-replicating RNA molecules proliferated before the evolution of DNA and proteins. The term also refers to the hypothesis that posits the existence of this stage.

Nucleotide bases are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases—adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called primary or canonical. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an extra amine group, creating a more stable bond to thymine.
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase and a five-carbon sugar whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building blocks of DNA and RNA.

In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds.

Leslie Eleazer Orgel FRS was a British chemist. He is known for his theories on the origin of life.
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar, with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chains of nucleotides made through the processes of DNA replication and transcription. Nucleoside triphosphates also serve as a source of energy for cellular reactions and are involved in signalling pathways.
In molecular biology, a polynucleotide is a biopolymer composed of nucleotide monomers that are covalently bonded in a chain. DNA and RNA are examples of polynucleotides with distinct biological functions. DNA consists of two chains of polynucleotides, with each chain in the form of a helix.
Pyrimidine biosynthesis occurs both in the body and through organic synthesis.
A protocell is a self-organized, endogenously ordered, spherical collection of lipids proposed as a rudimentary precursor to cells during the origin of life. A central question in evolution is how simple protocells first arose and how their progeny could diversify, thus enabling the accumulation of novel biological emergences over time. Although a functional protocell has not yet been achieved in a laboratory setting, the goal to understand the process appears well within reach.
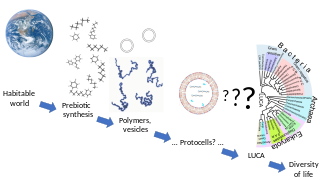
Abiogenesis is the natural process by which life arises from non-living matter, such as simple organic compounds. The prevailing scientific hypothesis is that the transition from non-living to living entities on Earth was not a single event, but a process of increasing complexity involving the formation of a habitable planet, the prebiotic synthesis of organic molecules, molecular self-replication, self-assembly, autocatalysis, and the emergence of cell membranes. The transition from non-life to life has never been observed experimentally, but many proposals have been made for different stages of the process.
James "Jim" P. Ferris was an American chemist. He is known for his contributions to the understanding of the origins of life on Earth, specifically by demonstrating a successful mechanism of clay-catalyzed polymerization of RNA, providing further evidence for the RNA World Hypothesis. Additionally, his work in atmospheric photochemistry has illuminated many of the chemical processes which occur in the atmospheres of Jupiter and Saturn's moon, Titan.
Formamide-based prebiotic chemistry is a reconstruction of the beginnings of life on Earth, assuming that formamide could accumulate in sufficiently high amounts to serve as the building block and reaction medium for the synthesis of the first biogenic molecules.
A scenario is a set of related concepts pertinent to the origin of life (abiogenesis), such as the iron-sulfur world. Many alternative abiogenesis scenarios have been proposed by scientists in a variety of fields from the 1950s onwards in an attempt to explain how the complex mechanisms of life could have come into existence. These include hypothesized ancient environments that might have been favourable for the origin of life, and possible biochemical mechanisms.
A proto-metabolism is a series of linked chemical reactions in a prebiotic environment that preceded and eventually turned into modern metabolism. Combining ongoing research in astrobiology and prebiotic chemistry, work in this area focuses on reconstructing the connections between potential metabolic processes that may have occurred in early Earth conditions. Proto-metabolism is believed to be simpler than modern metabolism and the Last Universal Common Ancestor (LUCA), as simple organic molecules likely gave rise to more complex metabolic networks. Prebiotic chemists have demonstrated abiotic generation of many simple organic molecules including amino acids, fatty acids, simple sugars, and nucleobases. There are multiple scenarios bridging prebiotic chemistry to early metabolic networks that occurred before the origins of life, also known as abiogenesis. In addition, there are hypotheses made on the evolution of biochemical pathways including the metabolism-first hypothesis, which theorizes how reaction networks dissipate free energy from which genetic molecules and proto-cell membranes later emerge. To determine the composition of key early metabolic networks, scientists have also used top-down approaches to study LUCA and modern metabolism.
A warm little pond is a hypothetical terrestrial shallow water environment on early Earth under which the origin of life could have occurred. The term was originally coined by Charles Darwin in an 1871 letter to his friend Joseph Dalton Hooker. This idea is related to later work such as the Oparin-Haldane hypothesis and the Miller–Urey experiment, which respectively provided a hypothesis for life’s origin from a primordial soup of organics and a proof of concept for the mechanism by which biomolecules and their precursors may have formed.
Cyanosulfidic prebiotic synthesis is a proposed mechanism for the origin of the key chemical building blocks of life. It involves a systems chemistry approach to synthesize the precursors of amino acids, ribonucleotides, and lipids using the same starting reagents and largely the same plausible early Earth conditions. Cyanosulfidic prebotic synthesis was developed by John Sutherland and co-workers at the Laboratory of Molecular Biology in Cambridge, England.








