Related Research Articles

Prostate stem cell antigen is a protein that in humans is encoded by the PSCA gene.

Homeobox protein Hox-B13 is a protein that in humans is encoded by the HOXB13 gene.

Arul M. Chinnaiyan is a Hicks Endowed Professor of Pathology and professor of pathology and urology at the University of Michigan Medical School. He is also a Howard Hughes medical Investigator (HHMI) at the Howard Hughes Medical Institute.
Douglas S. Scherr, M.D. is an American surgeon and specialist in Urologic Oncology. He is currently the Clinical Director of Urologic Oncology at Weill Cornell Medicine. He also holds an appointment at the Rockefeller University as a Visiting Associate Physician. Scherr was the first physician at Cornell to perform a robotic prostatectomy as well as a robotic cystectomy.
James L. Gulley is an American cancer researcher and the Director of the Medical Oncology Service at National Cancer Institute.

Charles L. Sawyers is a Howard Hughes Medical Institute (HHMI) investigator who holds the Marie-Josée and Henry R. Kravis Chair of the Human Oncology and Pathogenesis Program (HOPP) at Memorial Sloan Kettering Cancer Center (MSK). HOPP is a program created in 2006 that comprises researchers from many disciplines to bridge clinical and laboratory discoveries.
William K. Oh, is an American medical oncologist, academic and industry leader and expert in the management of genitourinary malignancies, including prostate, renal, bladder and testicular cancers.
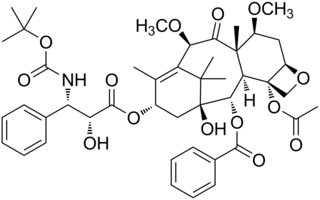
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.

Forkhead box protein A1 (FOXA1), also known as hepatocyte nuclear factor 3-alpha (HNF-3A), is a protein that in humans is encoded by the FOXA1 gene.
William Douglas Figg is an American scientist (pharmacologist). He is a senior investigator (tenured) at the National Cancer Institute (NCI), National Institutes of Health (NIH), Bethesda, Maryland. He holds multiple titles within the NCI: Associate Director of the Center for Cancer Research, Co-Director of the Office of Translational Resources, Acting Branch Chief for the Genitourinary Malignancies Branch, Chief of the Clinical Pharmacology Program, and head of the Molecular Pharmacology Section. Dr. Figg is also the Co-Chief of Basic Research at the Center for Prostate Disease Research within the Walter Reed National Military Medical Center – Murtha Cancer Center in Bethesda, Maryland.
EPI-001 is the first inhibitor of the androgen receptor amino-terminal domain. The single stereoisomer of EPI-001, EPI-002, is a first-in-class drug that the USAN council assigned a new stem class "-aniten" and the generic name "ralaniten". This distinguishes the anitens novel molecular mechanism from anti androgens that bind the C-terminus ligand-binding domain and have the stem class "lutamide". EPI-001 and its stereoisomers and analogues were discovered by Marianne Sadar and Raymond Andersen, who co-founded the pharmaceutical company ESSA Pharma Inc for the clinical development of anitens for the treatment of castration-resistant prostate cancer (CRPC).
Waun Ki Hong was Professor of Medicine at The University of Texas MD Anderson Cancer Center, where he served as chairman of the Department of Thoracic/Head & Neck Medical Oncology from 1993 to 2005 and as head of the Division of Cancer Medicine from 2001 to 2014. He was also an American Cancer Society Professor and the Samsung Distinguished University Chair in Cancer Medicine emeritus.

Apricoxib is an experimental anticancer drug and nonsteroidal anti-inflammatory drug (NSAID). It is a COX-2 inhibitor which is intended to improve standard therapy response in molecularly-defined models of pancreatic cancer. It was also studied in clinical trials for non-small-cell lung cancer. Development was abandoned in 2015 due to poor clinical trial results.

Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.

Dannielle Engle is an American biologist and assistant professor of the regulatory biology laboratory at the Salk Institute for Biological Studies. Engle’s research aims at improving detection and treatment of pancreatic cancer.
Urinary cell-free DNA (ucfDNA) refers to DNA fragments in urine released by urogenital and non-urogenital cells. Shed cells on urogenital tract release high- or low-molecular-weight DNA fragments via apoptosis and necrosis, while circulating cell-free DNA (cfDNA) that passes through glomerular pores contributes to low-molecular-weight DNA. Most of the ucfDNA is low-molecular-weight DNA in the size of 150-250 base pairs. The detection of ucfDNA composition allows the quantification of cfDNA, circulating tumour DNA, and cell-free fetal DNA components. Many commercial kits and devices have been developed for ucfDNA isolation, quantification, and quality assessment.

Alberto Bardelli is an Italian geneticist and cancer researcher, expert in the field of precision medicine. He is a full professor of histology at the Department of Oncology, University of Turin and Scientific Director of IFOM, the AIRC Institute of Molecular Oncology.
Richard B. Gaynor is an American physician specializing in hematology-oncology, educator, drug developer, and business executive. He served as an Associate Professor of Medicine at UCLA School of Medicine for nearly a decade, and subsequently as an endowed Professor of Medicine and Microbiology at the University of Texas Southwestern Medical School prior to joining the pharmaceutical industry in 2002. His research on NF-κB, IκB kinase, and other mechanisms regulating viral and cellular gene expression has been covered in leading subject reviews. He has been a top executive at several pharmaceutical companies, with respect to the development and clinical testing of novel anticancer drugs and cell therapies. For over a decade and a half, he worked at Eli Lilly and Company, where he became the Senior Vice President of Oncology Clinical Development and Medical Affairs in 2013. Gaynor was President of R&D at Neon Therapeutics from 2016 to 2020, when he became the President of BioNTech US, both pharmaceutical companies headquartered in Cambridge, MA. His honors include being elected a member of the American Society for Clinical Investigation, and the Association of American Physicians.
Lisa M. Coussens is an American cancer scientist who is Chair of the Department of Cell, Developmental and Cancer Biology and Professor and Associate Director for Basic Research in the Knight Cancer Institute at the Oregon Health & Science University. She serves as President of the American Association for Cancer Research.

Inavolisib, or GDC-0077, is an early-phase, highly selective inhibitor and degrader of mutant phosphatidylinositol 3-kinase (PI3K) alpha. The PI3K-mediated signalling pathway has shown to play an important role in the development of tumours as dysregulation is commonly associated with tumour growth and resistance to antineoplastic agents and radiotherapy.
References
- ↑ "American Cancer Society Names Dr. Karen Knudsen as its Next CEO". cancer.org. American Cancer Society. Retrieved 8 June 2021.
- ↑ "Editorial Board | Molecular Cancer Research". mcr.aacrjournals.org. Retrieved 2020-11-27.
- ↑ "100 Influential Women in Oncology: Key Opinion Leaders to follow on Social Media in 2023". OncoDaily.
- ↑ Sharma, Ankur; Comstock, Clay E. S.; Knudsen, Erik S.; Cao, Khanh H.; Hess-Wilson, Janet K.; Morey, Lisa M.; Barrera, Jason; Knudsen, Karen E. (2007-07-01). "Retinoblastoma Tumor Suppressor Status Is a Critical Determinant of Therapeutic Response in Prostate Cancer Cells". Cancer Research. 67 (13): 6192–6203. doi:10.1158/0008-5472.CAN-06-4424. ISSN 0008-5472. PMC 4133940 . PMID 17616676.
- ↑ Sharma, Ankur; Yeow, Wen-Shuz; Ertel, Adam; Coleman, Ilsa; Clegg, Nigel; Thangavel, Chellappagounder; Morrissey, Colm; Zhang, Xiaotun; Comstock, Clay E. S.; Witkiewicz, Agnieszka K.; Gomella, Leonard (December 2010). "The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression". The Journal of Clinical Investigation. 120 (12): 4478–4492. doi:10.1172/JCI44239. ISSN 1558-8238. PMC 2993601 . PMID 21099110.
- ↑ McNair, Christopher; Xu, Kexin; Mandigo, Amy C.; Benelli, Matteo; Leiby, Benjamin; Rodrigues, Daniel; Lindberg, Johan; Gronberg, Henrik; Crespo, Mateus; De Laere, Bram; Dirix, Luc (January 2018). "Differential impact of RB status on E2F1 reprogramming in human cancer". The Journal of Clinical Investigation. 128 (1): 341–358. doi:10.1172/JCI93566. ISSN 1558-8238. PMC 5749518 . PMID 29202480.
- ↑ Goodwin, Jonathan F.; Schiewer, Matthew J.; Dean, Jeffry L.; Schrecengost, Randy S.; de Leeuw, Renée; Han, Sumin; Ma, Teng; Den, Robert B.; Dicker, Adam P.; Feng, Felix Y.; Knudsen, Karen E. (November 2013). "A hormone-DNA repair circuit governs the response to genotoxic insult". Cancer Discovery. 3 (11): 1254–1271. doi:10.1158/2159-8290.CD-13-0108. ISSN 2159-8290. PMC 3823813 . PMID 24027197.
- ↑ Goodwin, Jonathan F.; Kothari, Vishal; Drake, Justin M.; Zhao, Shuang; Dylgjeri, Emanuela; Dean, Jeffry L.; Schiewer, Matthew J.; McNair, Christopher; Jones, Jennifer K.; Aytes, Alvaro; Magee, Michael S. (2015-07-13). "DNA-PKcs-Mediated Transcriptional Regulation Drives Prostate Cancer Progression and Metastasis". Cancer Cell. 28 (1): 97–113. doi:10.1016/j.ccell.2015.06.004. ISSN 1878-3686. PMC 4531387 . PMID 26175416.
- ↑ Dylgjeri, Emanuela; McNair, Christopher; Goodwin, Jonathan F.; Raymon, Heather K.; McCue, Peter A.; Shafi, Ayesha A.; Leiby, Benjamin E.; Leeuw, Renée de; Kothari, Vishal; McCann, Jennifer J.; Mandigo, Amy C. (2019-09-15). "Pleiotropic Impact of DNA-PK in Cancer and Implications for Therapeutic Strategies". Clinical Cancer Research. 25 (18): 5623–5637. doi:10.1158/1078-0432.CCR-18-2207. ISSN 1078-0432. PMC 6744985 . PMID 31266833.
- ↑ Burd, Craig J.; Petre, Christin E.; Morey, Lisa M.; Wang, Ying; Revelo, Monica P.; Haiman, Christopher A.; Lu, Shan; Fenoglio-Preiser, Cecilia M.; Li, Jiwen; Knudsen, Erik S.; Wong, Jiemin (2006-02-14). "Cyclin D1b variant influences prostate cancer growth through aberrant androgen receptor regulation". Proceedings of the National Academy of Sciences. 103 (7): 2190–2195. Bibcode:2006PNAS..103.2190B. doi: 10.1073/pnas.0506281103 . ISSN 0027-8424. PMC 1413684 . PMID 16461912.
- ↑ Augello, Michael A.; Burd, Craig J.; Birbe, Ruth; McNair, Christopher; Ertel, Adam; Magee, Michael S.; Frigo, Daniel E.; Wilder-Romans, Kari; Shilkrut, Mark; Han, Sumin; Jernigan, Danielle L. (2013-01-02). "Convergence of oncogenic and hormone receptor pathways promotes metastatic phenotypes". The Journal of Clinical Investigation. 123 (1): 493–508. doi:10.1172/JCI64750. ISSN 0021-9738. PMC 3533295 . PMID 23257359.
- ↑ de Leeuw, Renée; McNair, Christopher; Schiewer, Matthew J.; Neupane, Neermala Poudel; Brand, Lucas J.; Augello, Michael A.; Li, Zhen; Cheng, Larry C.; Yoshida, Akihiro; Courtney, Sean M.; Hazard, E. Starr (September 2018). "MAPK Reliance via Acquired CDK4/6 Inhibitor Resistance in Cancer". Clinical Cancer Research. 24 (17): 4201–4214. doi:10.1158/1078-0432.CCR-18-0410. ISSN 1078-0432. PMC 6125187 . PMID 29739788.
- ↑ Schiewer, Matthew J.; Goodwin, Jonathan F.; Han, Sumin; Brenner, J. Chad; Augello, Michael A.; Dean, Jeffry L.; Liu, Fengzhi; Planck, Jamie L.; Ravindranathan, Preethi; Chinnaiyan, Arul M.; McCue, Peter (December 2012). "Dual roles of PARP-1 promote cancer growth and progression". Cancer Discovery. 2 (12): 1134–1149. doi:10.1158/2159-8290.CD-12-0120. ISSN 2159-8290. PMC 3519969 . PMID 22993403.
- ↑ Feng, Felix Y.; de Bono, Johann S.; Rubin, Mark A.; Knudsen, Karen E. (2015-06-18). "Chromatin to Clinic: The Molecular Rationale for PARP1 Inhibitor Function". Molecular Cell. 58 (6): 925–934. doi:10.1016/j.molcel.2015.04.016. ISSN 1097-4164. PMC 4487541 . PMID 26091341.
- ↑ Schiewer, Matthew J.; Mandigo, Amy C.; Gordon, Nicolas; Huang, Fangjin; Gaur, Sanchaika; de Leeuw, Renée; Zhao, Shuang G.; Evans, Joseph; Han, Sumin; Parsons, Theodore; Birbe, Ruth (1 December 2018). "PARP-1 regulates DNA repair factor availability". EMBO Molecular Medicine. 10 (12). doi:10.15252/emmm.201708816. ISSN 1757-4684. PMC 6284389 . PMID 30467127.
- ↑ Research, Center for Drug Evaluation and (2020-05-20). "FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer". FDA.
- ↑ "AACI President Knudsen to Focus Two-Year Term on Mitigating Health Disparities". www.newswise.com. Retrieved 2021-01-08.
- ↑ "Board of Directors | AACR About Us". American Association for Cancer Research (AACR). Retrieved 2021-01-08.
- ↑ "NCI Board of Scientific Advisors". deainfo.nci.nih.gov. Retrieved 2020-11-27.
- ↑ "Genentech: Scientific Research Board". www.gene.com. Retrieved 2020-11-27.
- ↑ "Editorial Board | Molecular Cancer Research". mcr.aacrjournals.org. Retrieved 2020-11-27.
- ↑ "About the Editors | Oncogene". www.nature.com. Retrieved 2020-11-27.