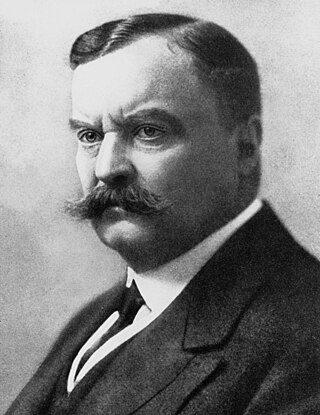
African trypanosomiasis, also known as African sleeping sickness or simply sleeping sickness, is an insect-borne parasitic infection of humans and other animals. It is caused by the species Trypanosoma brucei. Humans are infected by two types, Trypanosoma brucei gambiense (TbG) and Trypanosoma brucei rhodesiense (TbR). TbG causes over 98% of reported cases. Both are usually transmitted by the bite of an infected tsetse fly and are most common in rural areas.

Trypanosomatida is a group of kinetoplastid excavates distinguished by having only a single flagellum. The name is derived from the Greek trypano (borer) and soma (body) because of the corkscrew-like motion of some trypanosomatid species. All members are exclusively parasitic, found primarily in insects. A few genera have life-cycles involving a secondary host, which may be a vertebrate, invertebrate or plant. These include several species that cause major diseases in humans. Some trypanosomatida are intracellular parasites, with the important exception of Trypanosoma brucei.

Tsetse, are large, biting flies that inhabit much of tropical Africa. Tsetse flies include all the species in the genus Glossina, which are placed in their own family, Glossinidae. The tsetse is an obligate parasite, which lives by feeding on the blood of vertebrate animals. Tsetse has been extensively studied, because of their role in transmitting disease. They have a prominent economic impact in sub-Saharan Africa, as the biological vectors of trypanosomes, causing human and animal trypanosomiasis.

Trypanosomiasis or trypanosomosis is the name of several diseases in vertebrates caused by parasitic protozoan trypanosomes of the genus Trypanosoma. In humans this includes African trypanosomiasis and Chagas disease. A number of other diseases occur in other animals.

Trypanosoma is a genus of kinetoplastids, a monophyletic group of unicellular parasitic flagellate protozoa. Trypanosoma is part of the phylum Sarcomastigophora. The name is derived from the Greek trypano- (borer) and soma (body) because of their corkscrew-like motion. Most trypanosomes are heteroxenous and most are transmitted via a vector. The majority of species are transmitted by blood-feeding invertebrates, but there are different mechanisms among the varying species. Some, such as Trypanosoma equiperdum, are spread by direct contact. In an invertebrate host they are generally found in the intestine, but normally occupy the bloodstream or an intracellular environment in the vertebrate host.

Melarsoprol is an arsenic-containing medication used for the treatment of sleeping sickness. It is specifically used for second-stage disease caused by Trypanosoma brucei rhodesiense when the central nervous system is involved. For Trypanosoma brucei gambiense, eflornithine or fexinidazole is usually preferred. It is effective in about 95% of people. It is given by injection into a vein.
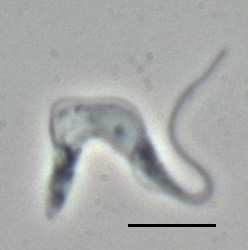
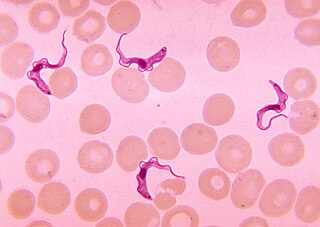
Trypanosoma brucei is a species of parasitic kinetoplastid belonging to the genus Trypanosoma that is present in sub-Saharan Africa. Unlike other protozoan parasites that normally infect blood and tissue cells, it is exclusively extracellular and inhabits the blood plasma and body fluids. It causes deadly vector-borne diseases: African trypanosomiasis or sleeping sickness in humans, and animal trypanosomiasis or nagana in cattle and horses. It is a species complex grouped into three subspecies: T. b. brucei, T. b. gambiense and T. b. rhodesiense. The first is a parasite of non-human mammals and causes nagana, while the latter two are zoonotic infecting both humans and animals and cause African trypanosomiasis.

Trypanosoma evansi is a parasitic species of excavate trypanosome in the genus Trypanosoma that is one cause of surra in animals. Discovered by Griffith Evans in 1880 at Dera Ismail Khan, it is the first known trypanosome that causes infection. It is a common parasite in India and Iran and causes acute disease in camels and horses, and chronic disease in cattle and buffalo. In Pakistan, it has been found to be the most prevalent trypanosome species in donkeys. It is now established to infect other mammals, including humans.
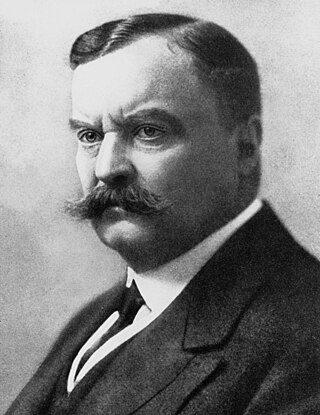
Major-General Sir David Bruce was a Scottish pathologist and microbiologist who made some of the key contributions in tropical medicine. In 1887, he discovered a bacterium, now called Brucella, that caused what was known as Malta fever. In 1894, he discovered a protozoan parasite, named Trypanosoma brucei, as the causative pathogen of nagana.

Animal trypanosomiasis, also known as nagana and nagana pest, or sleeping sickness, is a disease of vertebrates. The disease is caused by trypanosomes of several species in the genus Trypanosoma such as Trypanosoma brucei. Trypanosoma vivax causes nagana mainly in West Africa, although it has spread to South America. The trypanosomes infect the blood of the vertebrate host, causing fever, weakness, and lethargy, which lead to weight loss and anemia; in some animals the disease is fatal unless treated. The trypanosomes are transmitted by tsetse flies.
Antigenic variation or antigenic alteration refers to the mechanism by which an infectious agent such as a protozoan, bacterium or virus alters the proteins or carbohydrates on its surface and thus avoids a host immune response, making it one of the mechanisms of antigenic escape. It is related to phase variation. Antigenic variation not only enables the pathogen to avoid the immune response in its current host, but also allows re-infection of previously infected hosts. Immunity to re-infection is based on recognition of the antigens carried by the pathogen, which are "remembered" by the acquired immune response. If the pathogen's dominant antigen can be altered, the pathogen can then evade the host's acquired immune system. Antigenic variation can occur by altering a variety of surface molecules including proteins and carbohydrates. Antigenic variation can result from gene conversion, site-specific DNA inversions, hypermutation, or recombination of sequence cassettes. The result is that even a clonal population of pathogens expresses a heterogeneous phenotype. Many of the proteins known to show antigenic or phase variation are related to virulence.

Trypanosoma congolense is a species of trypanosomes and is the major pathogen responsible for the disease nagana in cattle and other animals including sheep, pigs, goats, horses and camels, dogs, as well as laboratory mice. It is the most common cause of nagana in east Africa, but is also a major cause of nagana in west Africa. This parasite is spread by tsetse flies. In its mammalian host, Trypanosoma congolense only lives in blood vessels, and causes in particular anaemia.
Wendy Gibson is Professor of Protozoology at University of Bristol, specialising in trypanosomes and molecular parasitology.

Trypanosoma lewisi is a globally distributed parasite of Rattus species and other rodents such as mice, and of kangaroo rats in America. Among these host species were two endemic species of rats: Rattus macleari and Rattus nativitatis. Both are now believed to be extinct. It is not very clear whether or not the same parasite infected both species. However, both parasites are very similar. The northern rat flea acts as the vector for the parasite, harboring the epimastigote stage in its midgut. The trypomastigote is the stage that is present in the main host, the rodent. The epimastigote form attaches itself to the rectum of the insect using its flagella to burrow through the rectal walls. The parasites also appear in the flea's feces. Ingestion of either the flea or its feces during grooming infects the host rodent with the parasites. T. lewisi is normally non-pathogenic but is known to have produced fatal infections in rats.
Procyclins also known as procyclic acidic repetitive proteins or PARP are proteins developed in the surface coating of Trypanosoma brucei parasites while in their tsetse fly vector. The cell surface of the bloodstream form features a dense coat of variable surface glycoproteins (VSGs) which is replaced by an equally dense coat of procyclins when the parasite differentiates into the procylic form in the tsetse fly midgut.

Variant surface glycoprotein (VSG) is a ~60kDa protein which densely packs the cell surface of protozoan parasites belonging to the genus Trypanosoma. This genus is notable for their cell surface proteins. They were first isolated from Trypanosoma brucei in 1975 by George Cross. VSG allows the trypanosomatid parasites to evade the mammalian host's immune system by extensive antigenic variation. They form a 12–15 nm surface coat. VSG dimers, ~90% of all cell surface protein. It also makes up ~10% of total cell protein. For this reason, these proteins are highly immunogenic and an immune response raised against a specific VSG coat will rapidly kill trypanosomes expressing this variant. However, with each cell division there is a possibility that the progeny will switch expression to change the VSG that is being expressed. VSG has no prescribed biochemical activity.

John William Watson Stephens FRS (1865–1946) was a British parasitologist and expert on tropical diseases.
David Horn FRSE, is a Wellcome Trust Senior Investigator, professor of parasite molecular biology, deputy head of the Division of Biological Chemistry and Drug Discovery and deputy director of the Wellcome Trust Centre for Anti-Infectives Research in the School of Life Sciences, University of Dundee. His research is focused on antigenic variation, drug action and resistance and the application of genetic screens to African trypanosomes: parasitic protists that cause sleeping sickness or Human African Trypanosomiasis (HAT) and the livestock disease, nagana.
The Sleeping Sickness Commission was a medical project established by the British Royal Society to investigate the outbreak of African sleeping sickness or African trypanosomiasis in Africa at the turn of the 20th century. The outbreak of the disease started in 1900 in Uganda, which was at the time a protectorate of the British Empire. The initial team in 1902 consisted of Aldo Castellani and George Carmichael Low, both from the London School of Hygiene and Tropical Medicine, and Cuthbert Christy, a medical officer on duty in Bombay, India. From 1903, David Bruce of the Royal Army Medical Corps and David Nunes Nabarro of the University College Hospital took over the leadership. The commission established that species of blood protozoan called Trypanosoma brucei, named after Bruce, was the causative parasite of sleeping sickness.
Fatma Serap Aksoy is a Turkish–American medical entomologist.