Crimson is a rich, deep red color, inclining to purple. It originally meant the color of the kermes dye produced from a scale insect, Kermes vermilio, but the name is now sometimes also used as a generic term for slightly bluish-red colors that are between red and rose. It is the national color of Nepal.

Carmine – also called cochineal, cochineal extract, crimson lake, or carmine lake – is a pigment of a bright-red color obtained from the aluminium complex derived from carminic acid. Specific code names for the pigment include natural red 4, C.I. 75470, or E120. Carmine is also a general term for a particularly deep-red color.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
A lake pigment is a pigment made by precipitating a dye with an inert binder, or mordant, usually a metallic salt. Lake pigments are largely chemically organic. Manufacturers and suppliers to artists and industry frequently omit the lake designation in the name. Many lake pigments are fugitive because the dyes involved are not lightfast. Red lakes were particularly important in Renaissance and Baroque paintings; they were often used as translucent glazes to portray the colors of rich fabrics and draperies.

Carminic acid (C22H20O13) is a red glucosidal hydroxyanthrapurin that occurs naturally in some scale insects, such as the cochineal, Armenian cochineal, and Polish cochineal. The insects produce the acid as a deterrent to predators. An aluminum salt of carminic acid is the coloring agent in carmine, a pigment. Natives of Peru had been producing cochineal dyes for textiles since at least 700 CE. Synonyms are C.I. 75470 and C.I. Natural Red 4.

Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula C6H4(OH)2. It is the ortho isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Small amounts of catechol occur in fruits and vegetables.

Aurin, sometimes named rosolic acid or corallin is an organic compound, forming yellowish or deep-red crystals with greenish metallic luster. It is practically insoluble in water, freely soluble in alcohol. It is soluble in strong acids to form yellow solution, or in aqueous alkalis to form carmine red solutions.

In organic chemistry, aromatic sulfonation is an reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid group. Together with nitration and chlorination, aromatic sulfonation is a widely used electrophilic aromatic substitutions. Aryl sulfonic acids are used as detergents, dye, and drugs.

Kermes is a red dye derived from the dried bodies of the females of a scale insect in the genus Kermes, primarily Kermes vermilio. The Kermes insects are native in the Mediterranean region and are parasites living on the sap of the host plant, the Kermes oak and the Palestine oak.

For the parent molecule 9,10-anthraquinone, see anthraquinone

Polish cochineal, also known as Polish carmine scales, is a scale insect formerly used to produce a crimson dye of the same name, colloquially known as "Saint John's blood". The larvae of P. polonica are sessile parasites living on the roots of various herbs – especially those of the perennial knawel – growing on the sandy soils of Central Europe and other parts of Eurasia. Before the development of aniline, alizarin, and other synthetic dyes, the insect was of great economic importance, although its use was in decline after the introduction of Mexican cochineal to Europe in the 16th century. Historically earlier was used Armenian cochineal scale insect, Porphyrophora hamelii, which is in a same taxonomic family Porphyrophora of the Polish cochineal and in different taxonomic family from cochineal found in the Americas.
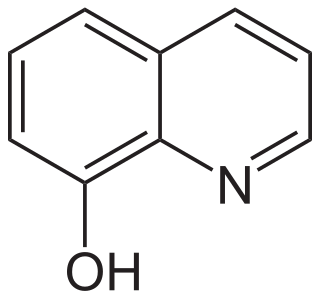
8-Hydroxyquinoline is an organic compound derived from the heterocycle quinoline. A colorless solid, its conjugate base is a chelating agent, which is used for the quantitative determination of metal ions.

Otto Dimroth was a German chemist. He is known for the Dimroth rearrangement, as well as a type of condenser with an internal double spiral, the Dimroth condenser.
The Dimroth rearrangement is a rearrangement reaction taking place with certain 1,2,3-triazoles where endocyclic and exocyclic nitrogen atoms switch place. This organic reaction was discovered in 1909 by Otto Dimroth.
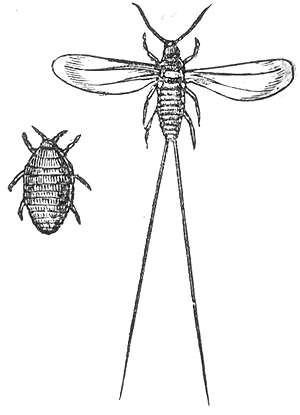
The cochineal is a scale insect in the suborder Sternorrhyncha, from which the natural dye carmine is derived. A primarily sessile parasite native to tropical and subtropical South America through North America, this insect lives on cacti in the genus Opuntia, feeding on plant moisture and nutrients. The insects are found on the pads of prickly pear cacti, collected by brushing them off the plants, and dried.

Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes.

Dyeing is the craft of imparting colors to textiles in loose fiber, yarn, cloth or garment form by treatment with a dye. Archaeologists have found evidence of textile dyeing with natural dyes dating back to the Neolithic period. In China, dyeing with plants, barks and insects has been traced back more than 5,000 years. Natural insect dyes such as Tyrian purple and kermes and plant-based dyes such as woad, indigo and madder were important elements of the economies of Asia and Europe until the discovery of man-made synthetic dyes in the mid-19th century. Synthetic dyes quickly superseded natural dyes for the large-scale commercial textile production enabled by the Industrial Revolution, but natural dyes remained in use by traditional cultures around the world.
The Bohn–Schmidt reaction, a named reaction in chemistry, introduces a hydroxy group at an anthraquinone system. The anthraquinone must already have at least one hydroxy group. The reaction was first described in 1889 by René Bohn (1862–1922) and in 1891 by Robert Emanuel Schmidt (1864–1938), two German industrial chemists.

The Armenian cochineal, also known as the Ararat cochineal or Ararat scale, is a scale insect indigenous to the Ararat plain and Aras (Araks) River valley in the Armenian Highlands, including East of Turkey. It was formerly used to produce an eponymous crimson carmine dyestuff known in Armenia as vordan karmir and historically in Persia as kirmiz. The species is critically endangered within Armenia.
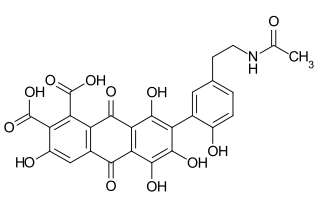
Laccaic acids or laccainic acids are a group of five anthraquinone derivatives, designated A through E, which are components of the red shellac obtained from the insect Kerria lacca, similar to carminic acid and kermesic acid. This article focuses primarily on laccaic acid A (LCA).















