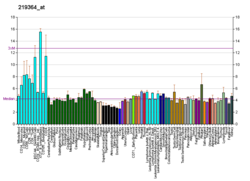
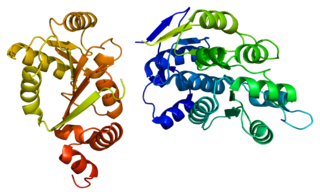Helicases are a class of enzymes thought to be vital to all organisms. Their main function is to unpack an organism's genetic material. Helicases are motor proteins that move directionally along a nucleic acid phosphodiester backbone, separating two hybridized nucleic acid strands, using energy from ATP hydrolysis. There are many helicases, representing the great variety of processes in which strand separation must be catalyzed. Approximately 1% of eukaryotic genes code for helicases.
Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells. It may also refer to other methods and cell types, although other terms are often preferred: "transformation" is typically used to describe non-viral DNA transfer in bacteria and non-animal eukaryotic cells, including plant cells. In animal cells, transfection is the preferred term as transformation is also used to refer to progression to a cancerous state (carcinogenesis) in these cells. Transduction is often used to describe virus-mediated gene transfer into eukaryotic cells.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

Toll-like receptor 3 (TLR3) also known as CD283 is a protein that in humans is encoded by the TLR3 gene. TLR3 is a member of the toll-like receptor family of pattern recognition receptors of the innate immune system. TLR3 recognizes double-stranded RNA in endosomes, which is a common feature of viral genomes internalised by macrophages and dendritic cells.

Ribonuclease L or RNase L, known sometimes as ribonuclease 4 or 2'-5' oligoadenylate synthetase-dependent ribonuclease, is an interferon (IFN)-induced ribonuclease which, upon activation, destroys all RNA within the cell. RNase L is an enzyme that in humans is encoded by the RNASEL gene.

RIG-I is a cytosolic pattern recognition receptor (PRR) that can mediate induction of a type-I interferon (IFN1) response. RIG-I is an essential molecule in the innate immune system for recognizing cells that have been infected with a virus. These viruses can include West Nile virus, Japanese Encephalitis virus, influenza A, Sendai virus, flavivirus, and coronaviruses.

ATP-dependent RNA helicase A is an enzyme that in humans is encoded by the DHX9 gene.
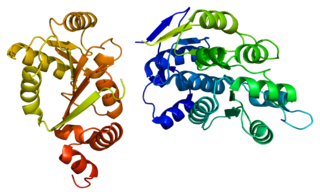
ATP-dependent RNA helicase DDX3X is an enzyme that in humans is encoded by the DDX3X gene.

Retinoic acid receptor responder protein 3 is a protein that in humans is encoded by the RARRES3 gene.

Mitochondrial antiviral-signaling protein (MAVS) is a protein that is essential for antiviral innate immunity. MAVS is located in the outer membrane of the mitochondria, peroxisomes, and mitochondrial-associated endoplasmic reticulum membrane (MAM). Upon viral infection, a group of cytosolic proteins will detect the presence of the virus and bind to MAVS, thereby activating MAVS. The activation of MAVS leads the virally infected cell to secrete cytokines. This induces an immune response which kills the host's virally infected cells, resulting in clearance of the virus.

Helicase SKI2W is an enzyme that in humans is encoded by the SKIV2L gene. This enzyme is a human homologue of yeast SKI2, which may be involved in antiviral activity by blocking translation of poly(A) deficient mRNAs. The SKIV2L gene is located in the class III region of the major histocompatibility complex.

Tripartite motif-containing protein 25 is a protein that in humans is encoded by the TRIM25 gene.

MDA5 is a RIG-I-like receptor dsRNA helicase enzyme that is encoded by the IFIH1 gene in humans. MDA5 is part of the RIG-I-like receptor (RLR) family, which also includes RIG-I and LGP2, and functions as a pattern recognition receptor capable of detecting viruses. It is generally believed that MDA5 recognizes double stranded RNA (dsRNA) over 2000nts in length, however it has been shown that whilst MDA5 can detect and bind to cytoplasmic dsRNA, it is also activated by a high molecular weight RNA complex composed of ssRNA and dsRNA. For many viruses, effective MDA5-mediated antiviral responses are dependent on functionally active LGP2. The signaling cascades in MDA5 is initiated via CARD domain. Some observations made in cancer cells show that MDA5 also interacts with cellular RNA is able to induce an autoinflammatory response.

Probable ATP-dependent RNA helicase DHX36 also known as DEAH box protein 36 (DHX36) or MLE-like protein 1 (MLEL1) or G4 resolvase 1 (G4R1) or RNA helicase associated with AU-rich elements (RHAU) is an enzyme that in humans is encoded by the DHX36 gene.

Putative pre-mRNA-splicing factor ATP-dependent RNA helicase DHX15 is an enzyme that in humans is encoded by the DHX15 gene.

Putative pre-mRNA-splicing factor ATP-dependent RNA helicase DHX32 is an enzyme that in humans is encoded by the DHX32 gene.

Putative pre-mRNA-splicing factor ATP-dependent RNA helicase DHX16 is an enzyme that in humans is encoded by the DHX16 gene.
RIG-I-like receptors are a type of intracellular pattern recognition receptor involved in the recognition of viruses by the innate immune system. RIG-I is the best characterized receptor within the RIG-I like receptor (RLR) family. Together with MDA5 and LGP2, this family of cytoplasmic pattern recognition receptors (PRRs) are sentinels for intracellular viral RNA that is a product of viral infection. The RLR receptors provide frontline defence against viral infections in most tissues.

Stimulator of interferon genes (STING), also known as transmembrane protein 173 (TMEM173) and MPYS/MITA/ERIS is a protein that in humans is encoded by the STING1 gene.

DExH-box helicase 29 (DHX29) is a 155 kDa protein that in humans is encoded by the DHX29 gene.