DNA ligase 4 is an enzyme that in humans is encoded by the LIG4 gene. [5]
DNA ligase 4 is an enzyme that in humans is encoded by the LIG4 gene. [5]
The protein encoded by this gene is an ATP-dependent DNA ligase that joins double-strand breaks during the non-homologous end joining pathway of double-strand break repair. It is also essential for V(D)J recombination. Lig4 forms a complex with XRCC4, and further interacts with the DNA-dependent protein kinase (DNA-PK) and XLF/Cernunnos, which are also required for NHEJ. The crystal structure of the Lig4/XRCC4 complex has been resolved. [6] Defects in this gene are the cause of LIG4 syndrome. The yeast homolog of Lig4 is Dnl4.
In humans, deficiency of DNA ligase 4 results in a clinical condition known as LIG4 syndrome. This syndrome is characterized by cellular radiation sensitivity, growth retardation, developmental delay, microcephaly, facial dysmorphisms, increased disposition to leukemia, variable degrees of immunodeficiency and reduced number of blood cells. [7] [8]
Accumulation of DNA damage leading to stem cell exhaustion is regarded as an important aspect of aging. [9] [10] Deficiency of lig4 in pluripotent stem cells impairs Non-homologous end joining (NHEJ) and results in accumulation of DNA double-strand breaks and enhanced apoptosis. [8] Lig4 deficiency in the mouse causes a progressive loss of haematopoietic stem cells and bone marrow cellularity during aging. [11] The sensitivity of haematopoietic stem cells to lig4 deficiency suggests that lig4-mediated NHEJ is a key determinant of the ability of stem cells to maintain themselves against physiological stress over time. [8] [11]
LIG4 has been shown to interact with XRCC4 via its BRCT domain. [12] [6] This interaction stabilizes LIG4 protein in cells; cells that are deficient for XRCC4, such as XR-1 cells, have reduced levels of LIG4. [13]
LIG4 is an ATP-dependent DNA ligase. LIG4 uses ATP to adenylate itself and then transfers the AMP group to the 5' phosphate of one DNA end. Nucleophilic attack by the 3' hydroxyl group of a second DNA end and release of AMP yield the ligation product. Adenylation of LIG4 is stimulated by XRCC4 and XLF. [14]

DNA ligase is a type of enzyme that facilitates the joining of DNA strands together by catalyzing the formation of a phosphodiester bond. It plays a role in repairing single-strand breaks in duplex DNA in living organisms, but some forms may specifically repair double-strand breaks. Single-strand breaks are repaired by DNA ligase using the complementary strand of the double helix as a template, with DNA ligase creating the final phosphodiester bond to fully repair the DNA.

DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA damage, resulting in tens of thousands of individual molecular lesions per cell per day. Many of these lesions cause structural damage to the DNA molecule and can alter or eliminate the cell's ability to transcribe the gene that the affected DNA encodes. Other lesions induce potentially harmful mutations in the cell's genome, which affect the survival of its daughter cells after it undergoes mitosis. As a consequence, the DNA repair process is constantly active as it responds to damage in the DNA structure. When normal repair processes fail, and when cellular apoptosis does not occur, irreparable DNA damage may occur. This can eventually lead to malignant tumors, or cancer as per the two-hit hypothesis.
RecQ helicase is a family of helicase enzymes initially found in Escherichia coli that has been shown to be important in genome maintenance. They function through catalyzing the reaction ATP + H2O → ADP + P and thus driving the unwinding of paired DNA and translocating in the 3' to 5' direction. These enzymes can also drive the reaction NTP + H2O → NDP + P to drive the unwinding of either DNA or RNA.

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the very first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.

Non-homologous end joining (NHEJ) is a pathway that repairs double-strand breaks in DNA. It is called "non-homologous" because the break ends are directly ligated without the need for a homologous template, in contrast to homology directed repair (HDR), which requires a homologous sequence to guide repair. NHEJ is active in both non-dividing and proliferating cells, while HDR is not readily accessible in non-dividing cells. The term "non-homologous end joining" was coined in 1996 by Moore and Haber.
V(D)J recombination is the mechanism of somatic recombination that occurs only in developing lymphocytes during the early stages of T and B cell maturation. It results in the highly diverse repertoire of antibodies/immunoglobulins and T cell receptors (TCRs) found in B cells and T cells, respectively. The process is a defining feature of the adaptive immune system.
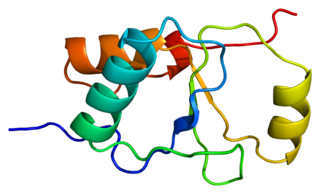
DNA repair protein XRCC1, also known as X-ray repair cross-complementing protein 1, is a protein that in humans is encoded by the XRCC1 gene. XRCC1 is involved in DNA repair, where it complexes with DNA ligase III.

DNA repair protein XRCC4 also known as X-ray repair cross-complementing protein 4 or XRCC4 is a protein that in humans is encoded by the XRCC4 gene. In addition to humans, the XRCC4 protein is also expressed in many other metazoans, fungi and in plants. The X-ray repair cross-complementing protein 4 is one of several core proteins involved in the non-homologous end joining (NHEJ) pathway to repair DNA double strand breaks (DSBs).

Ku70 is a protein that, in humans, is encoded by the XRCC6 gene.

Tumor suppressor p53-binding protein 1 also known as p53-binding protein 1 or 53BP1 is a protein that in humans is encoded by the TP53BP1 gene.

DNA polymerase mu is a polymerase enzyme found in eukaryotes. In humans, this protein is encoded by the POLM gene.
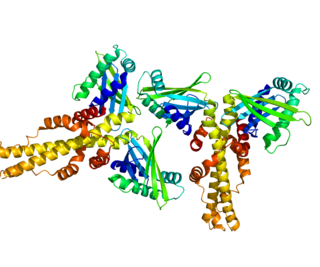
Non-homologous end-joining factor 1 (NHEJ1), also known as Cernunnos or XRCC4-like factor (XLF), is a protein that in humans is encoded by the NHEJ1 gene. XLF was originally discovered as the protein mutated in five patients with growth retardation, microcephaly, and immunodeficiency. The protein is required for the non-homologous end joining (NHEJ) pathway of DNA repair. Patients with XLF mutations also have immunodeficiency due to a defect in V(D)J recombination, which uses NHEJ to generate diversity in the antibody repertoire of the immune system. XLF interacts with DNA ligase IV and XRCC4 and is thought to be involved in the end-bridging or ligation steps of NHEJ. The yeast homolog of XLF is Nej1.
Microhomology-mediated end joining (MMEJ), also known as alternative nonhomologous end-joining (Alt-NHEJ) is one of the pathways for repairing double-strand breaks in DNA. As reviewed by McVey and Lee, the foremost distinguishing property of MMEJ is the use of microhomologous sequences during the alignment of broken ends before joining, thereby resulting in deletions flanking the original break. MMEJ is frequently associated with chromosome abnormalities such as deletions, translocations, inversions and other complex rearrangements.
The stem cell theory of aging postulates that the aging process is the result of the inability of various types of stem cells to continue to replenish the tissues of an organism with functional differentiated cells capable of maintaining that tissue's original function. Damage and error accumulation in genetic material is always a problem for systems regardless of the age. The number of stem cells in young people is very much higher than older people and thus creates a better and more efficient replacement mechanism in the young contrary to the old. In other words, aging is not a matter of the increase in damage, but a matter of failure to replace it due to a decreased number of stem cells. Stem cells decrease in number and tend to lose the ability to differentiate into progenies or lymphoid lineages and myeloid lineages.

LIG4 syndrome is an extremely rare condition caused by mutations in the DNA Ligase IV (LIG4) gene. Some mutations in this gene are associated with a resistance against multiple myeloma and Severe Combined Immunodeficiency. Severity of symptoms depends on the degree of reduced enzymatic activity of Ligase IV or gene expression. Ligase IV is a critical component of the non-homologous end joining (NHEJ) mechanism that repairs DNA double-strand breaks. It is employed in repairing DNA double-strand breaks caused by reactive oxygen species produced by normal metabolism, or by DNA damaging agents such as ionizing radiation. NHEJ is also used to repair the DNA double-strand break intermediates that occur in the production of T and B lymphocyte receptors.

Cernunnos deficiency is a form of combined immunodeficiency characterized by microcephaly, due to mutations in the NHEJ1 gene, it is inherited via autosomal recessive manner Management for this condition is antiviral prophylaxis and antibiotic treatment

DNA ligase 3 is an enzyme that, in humans, is encoded by the LIG3 gene. The human LIG3 gene encodes ATP-dependent DNA ligases that seal interruptions in the phosphodiester backbone of duplex DNA.
Penelope "Penny" Jeggo is a noted British molecular biologist, best known for her work in understanding damage to DNA. She is also known for her work with DNA gene mutations. Her interest in DNA damage has inspired her to research radiation biology and radiation therapy and how radiation affects DNA. Jeggo has more than 170 publications that pertain to DNA damage, radiation, and cancer research and has received 3 top science awards/medals for her research. Jeggo has also been a member of several organizations that pertain to radiation biology; these organizations include Committee on Medical Aspects of Radiation in the Environment (COMARE), National Institute for Radiation Science laboratory researcher, and the Multidisciplinary European Low Dose Initiative (MELODI). Jeggo is a member of these organizations, and she is also an editor for several publication journals that are related to cancer and radiation biology. Jeggo is very passionate about her research and in an interview with Fiona Watt claimed that “Although my results contributed only the tiniest smidgeon to scientific knowledge, I gained immense satisfaction from it”.

DNA end resection, also called 5′–3′ degradation, is a biochemical process where the blunt end of a section of double-stranded DNA (dsDNA) is modified by cutting away some nucleotides from the 5' end to produce a 3' single-stranded sequence. The presence of a section of single-stranded DNA (ssDNA) allows the broken end of the DNA to line up accurately with a matching sequence, so that it can be accurately repaired.

A double-strand break repair model refers to the various models of pathways that cells undertake to repair double strand-breaks (DSB). DSB repair is an important cellular process, as the accumulation of unrepaired DSB could lead to chromosomal rearrangements, tumorigenesis or even cell death. In human cells, there are two main DSB repair mechanisms: Homologous recombination (HR) and non-homologous end joining (NHEJ). HR relies on undamaged template DNA as reference to repair the DSB, resulting in the restoration of the original sequence. NHEJ modifies and ligates the damaged ends regardless of homology. In terms of DSB repair pathway choice, most mammalian cells appear to favor NHEJ rather than HR. This is because the employment of HR may lead to gene deletion or amplification in cells which contains repetitive sequences. In terms of repair models in the cell cycle, HR is only possible during the S and G2 phases, while NHEJ can occur throughout whole process. These repair pathways are all regulated by the overarching DNA damage response mechanism. Besides HR and NHEJ, there are also other repair models which exists in cells. Some are categorized under HR, such as synthesis-dependent strain annealing, break-induced replication, and single-strand annealing; while others are an entirely alternate repair model, namely, the pathway microhomology-mediated end joining (MMEJ).