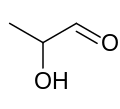
| |||
| Names | |||
|---|---|---|---|
| IUPAC name 2-Hydroxypropanal | |||
| Other names Hydroxypropionaldehyde | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.237.284 | ||
| KEGG | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C3H6O2 | |||
| Molar mass | 74.079 g·mol−1 | ||
| Related compounds | |||
Related aldehydes | Glycolaldehyde | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Lactaldehyde is an intermediate in the methylglyoxal metabolic pathway. Methylglyoxal is converted to D-lactaldehyde by glycerol dehydrogenase (gldA). Lactaldehyde is then oxidized to lactic acid by aldehyde dehydrogenase. [1]