Related Research Articles

Poliovirus, the causative agent of polio, is a serotype of the species Enterovirus C, in the family of Picornaviridae.

Rubella virus (RuV) is the pathogenic agent of the disease rubella, and is the main cause of congenital rubella syndrome when infection occurs during the first weeks of pregnancy.

Baculoviridae is a family of viruses. Arthropods, lepidoptera, hymenoptera, and diptera serve as natural hosts. There are currently 84 known species in this family, divided among four genera.
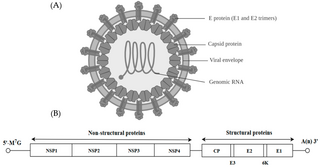
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 31 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.
The genome and proteins of HIV have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.
Jaagsiekte sheep retrovirus (JSRV) is a betaretrovirus which is the causative agent of a contagious lung cancer in sheep, called ovine pulmonary adenocarcinoma.
The murine leukemia viruses are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.
Visna virus from the genus Lentivirus and subfamily Orthoretrovirinae, is a "prototype" retrovirus that causes encephalitis and chronic pneumonitis in sheep. It is known as visna when found in the brain, and maedi when infecting the lungs. Lifelong, persistent infections in sheep occur in the lungs, lymph nodes, spleen, joints, central nervous system, and mammary glands; The condition is sometimes known as "ovine progressive pneumonia" (OPP), particularly in the United States, or "Montana sheep disease". White blood cells of the monocyte/macrophage lineage are the main target of visna virus.

Viral shedding refers to the expulsion and release of virus progeny following successful reproduction during a host-cell infection. Once replication has been completed and the host cell is exhausted of all resources in making viral progeny, the viruses may begin to leave the cell by several methods.
Avian sarcoma leukosis virus (ASLV) is an endogenous retrovirus that infects and can lead to cancer in chickens; experimentally it can infect other species of birds and mammals. ASLV replicates in chicken embryo fibroblasts, the cells that contribute to the formation of connective tissues. Different forms of the disease exist, including lymphoblastic, erythroblastic, and osteopetrotic.
Bovine immunodeficiency virus (BIV) is a retrovirus belonging to the genus Lentivirus. It is similar to the human immunodeficiency virus (HIV) and infects cattle. The cells primarily infected are lymphocytes and monocytes/macrophages.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.

Zaire ebolavirus, more commonly known as Ebola virus, is one of six known species within the genus Ebolavirus. Four of the six known ebolaviruses, including EBOV, cause a severe and often fatal hemorrhagic fever in humans and other mammals, known as Ebola virus disease (EVD). Ebola virus has caused the majority of human deaths from EVD, and was the cause of the 2013–2016 epidemic in western Africa, which resulted in at least 28,646 suspected cases and 11,323 confirmed deaths.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.
Entebbe bat virus is an infectious disease caused by a Flavivirus that is closely related to yellow fever.
Bovine foamy virus (BFV) is a ss(+)RNA retrovirus that belongs to the genus spumaviridae. Spumaviruses differ from the other six members of family retroviridae, both structurally and in pathogenic nature. Supmaviruses derive their name from spuma the latin for "foam". The 'foam' aspect of 'foamy virus' comes from syncytium formation and the rapid vacuolization of infected cells, creating a 'foamy' appearance.
Type 2 Porcine Reproductive and Respiratory Syndrome Virus is one type of the Porcine Reproductive and Respiratory Syndrome Viruses (PRRSV). The two types of PRRSV are distinguished by which genomic cluster they are associated with. Type 1 is associated with a LV cluster. Type 2 is associated with a VR2332 cluster. PRRSV is in the Arteriviridae family and the order Nidovirales. It has a positive sense RNA genome that is 15 kb long. This genome consists of ten open reading frames (ORFs) with a 5' untranslated region (UTR) and a 3' UTR. PRRSV causes Porcine Reproductive and Respiratory Syndrome in swine. This syndrome results in failure during breeding and respiratory problems. Type 2 PRRSV was first seen in the United States in 1987. However, it has now spread worldwide to commercial swine facilities.

Sepik virus (SEPV) is an arthropod-borne virus (arbovirus) of the genus Flavivirus and family Flaviviridae. Flaviviridae is one of the most well characterized viral families, as it contains many well-known viruses that cause diseases that have become very prevalent in the world, like Chikungunya virus and Dengue virus. The genus Flavivirus is one of the largest viral genera and encompasses over 50 viral species, including tick and mosquito borne viruses like Yellow fever virus and West Nile virus. Sepik virus is much less well known and has not been as well-classified as other viruses because it has not been known of for very long. Sepik virus was first isolated in 1966 from the mosquito Mansoniaseptempunctata, and it derives its name from the Sepik River area in Papua New Guinea, where it was first found. The geographic range of Sepik virus is limited to Papua New Guinea, due to its isolation.

Modoc virus (MODV) is a rodent-associated flavivirus. Small and enveloped, MODV contains positive single-stranded RNA. Taxonomically, MODV is part of the Flavivirus genus and Flaviviridae family. The Flavivirus genus includes nearly 80 viruses, both vector-borne and no known vector (NKV) species. Known flavivirus vector-borne viruses include Dengue virus, Yellow Fever virus, tick-borne encephalitis virus, and West Nile virus.
Rio Negro virus is an alphavirus that was first isolated in Argentina in 1980. The virus was first called Ag80-663 but was renamed to Rio Negro virus in 2005. The virus is a member of the Venezuelan equine encephalitis complex (VEEC), which are a group of alphaviruses in the Americas that have the potential to emerge and cause disease. Closely related viruses include Mucambo virus and Everglades virus.
References
- 1 2 3 Rowson, K.E.K.; B.W.J. Mahy (1985). "Lactate Dehydrogenase-Elevating Virus". Journal of General Virology. 66 ( Pt 11) (11): 2297–2312. doi: 10.1099/0022-1317-66-11-2297 . PMID 3903045. Archived from the original on 2008-08-21. Retrieved 2011-05-06.
- ↑ Mohammadi, Hakimeh; Shayan Sharif; Raymond R. Rowland; Dongwan Yon (11 June 2009). "The Lactate Dehydrogenase-Elevating Virus Capsid Protein is a Nuclear-Cytoplasmic Protein". Archives of Virology. 154 (7): 1071–1080. doi:10.1007/s00705-009-0410-0. PMC 7087266 . PMID 19517211.
- ↑ "Lactate Dehydrogenase Elevating Virus" (PDF). Charles River Technical Sheet. Archived from the original (PDF) on 2010-12-22. Retrieved 2011-05-06.
- 1 2 3 Anderson, Grant W.; Raymond R. R. Rowland; Gene A. Palmer; Chen Even; Peter G. W. Plagemann (August 1995). "Lactate Dehydrogenase-Elevating Virus Replication Persists in Liver, Spleen, Lymph Node, and Testis Tissues and Results in Accumulation of Viral RNA in Germinal Centers, Concomitant with Polycolonal Activation of B Cells". J. Virol. Journal of Virology. 69 (8): 5177–5185. doi:10.1128/JVI.69.8.5177-5185.1995. PMC 189342 . PMID 7609091.
- ↑ Brinton-Darnell, Margo; Peter G. W. Plagemann (August 1975). "Structure and Chemical-Physical Characteristics of Lactate Dehydrogenase-Elevating Virus and its RNA". J. Virol. Journal of Virology. 16 (2): 420–433. doi:10.1128/JVI.16.2.420-433.1975. PMC 354680 . PMID 1171266.