
Siboglinidae is a family of polychaete annelid worms whose members made up the former phyla Pogonophora and Vestimentifera. The family is composed of around 100 species of vermiform creatures which live in thin tubes buried in sediment (Pogonophora) or in tubes attached to hard substratum (Vestimentifera) at ocean depths ranging from 100 to 10,000 m. They can also be found in association with hydrothermal vents, methane seeps, sunken plant material, and whale carcasses.

Any worm that lives in a marine environment is considered a water worm. Marine worms are found in several different phyla, including the Platyhelminthes, Nematoda, Annelida, Chaetognatha, Hemichordata, and Phoronida. For a list of marine animals that have been called "sea worms", see sea worm.

Polychaeta is a paraphyletic class of generally marine annelid worms, commonly called bristle worms or polychaetes. Each body segment has a pair of fleshy protrusions called parapodia that bear many bristles, called chaetae, which are made of chitin. More than 10,000 species are described in this class. Common representatives include the lugworm and the sandworm or clam worm Alitta.

In biochemistry, chemosynthesis is the biological conversion of one or more carbon-containing molecules and nutrients into organic matter using the oxidation of inorganic compounds or ferrous ions as a source of energy, rather than sunlight, as in photosynthesis. Chemoautotrophs, organisms that obtain carbon from carbon dioxide through chemosynthesis, are phylogenetically diverse. Groups that include conspicuous or biogeochemically important taxa include the sulfur-oxidizing Gammaproteobacteria, the Campylobacterota, the Aquificota, the methanogenic archaea, and the neutrophilic iron-oxidizing bacteria.

A cold seep is an area of the ocean floor where seepage of fluids rich in hydrogen sulfide, methane, and other hydrocarbons occurs, often in the form of a brine pool. Cold does not mean that the temperature of the seepage is lower than that of the surrounding sea water; on the contrary, its temperature is often slightly higher. The "cold" is relative to the very warm conditions of a hydrothermal vent. Cold seeps constitute a biome supporting several endemic species.

Alvinella pompejana, the Pompeii worm, is a species of deep-sea polychaete worm. It is an extremophile found only at hydrothermal vents in the Pacific Ocean, discovered in the early 1980s off the Galápagos Islands by French marine biologists.

Riftia pachyptila, commonly known as the giant tube worm and less commonly known as the giant beardworm, is a marine invertebrate in the phylum Annelida related to tube worms commonly found in the intertidal and pelagic zones. R. pachyptila lives on the floor of the Pacific Ocean near hydrothermal vents. The vents provide a natural ambient temperature in their environment ranging from 2 to 30 °C, and this organism can tolerate extremely high hydrogen sulfide levels. These worms can reach a length of 3 m, and their tubular bodies have a diameter of 4 cm (1.6 in).
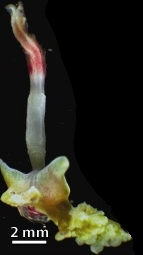
Osedax is a genus of deep-sea siboglinid polychaetes, commonly called boneworms, zombie worms, or bone-eating worms. Osedax is Latin for "bone-eater". The name alludes to how the worms bore into the bones of whale carcasses to reach enclosed lipids, on which they rely for sustenance. They utilize specialized root tissues for bone-boring. It is possible that multiple species of Osedax reside in the same bone. Osedax worms are also known to feed on the collagen itself by making holes in the whale's skeletal structure. These holes can also serve as a form of protection from nearby predators.

Lamellibrachia is a genus of tube worms related to the giant tube worm, Riftia pachyptila. They live at deep-sea cold seeps where hydrocarbons leak out of the seafloor, and are entirely reliant on internal, sulfide-oxidizing bacterial symbionts for their nutrition. The symbionts, gammaproteobacteria, require sulfide and inorganic carbon. The tube worms extract dissolved oxygen and hydrogen sulfide from the sea water with the crown of plumes. Species living near seeps can also obtain sulfide through their "roots", posterior extensions of their body and tube. Several sorts of hemoglobin are present in the blood and coelomic fluid to bind to the different components and transport them to the symbionts.
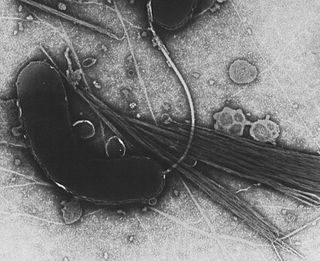
Gammaproteobacteria is a class of bacteria in the phylum Pseudomonadota. It contains about 250 genera, which makes it the most genus-rich taxon of the Prokaryotes. Several medically, ecologically, and scientifically important groups of bacteria belong to this class. All members of this class are Gram-negative. It is the most phylogenetically and physiologically diverse class of the Pseudomonadota.

Colleen Marie Cavanaugh is an American academic microbiologist best known for her studies of hydrothermal vent ecosystems. As of 2002, she is the Edward C. Jeffrey Professor of Biology in the Department of Organismic and Evolutionary Biology at Harvard University and is affiliated with the Marine Biological Laboratory and the Woods Hole Oceanographic Institution. Cavanaugh was the first to propose that the deep-sea giant tube worm, Riftia pachyptila, obtains its food from bacteria living within its cells, an insight which she had as a graduate student at Harvard. Significantly, she made the connection that these chemoautotrophic bacteria were able to play this role through their use of chemosynthesis, the biological oxidation of inorganic compounds to synthesize organic matter from very simple carbon-containing molecules, thus allowing organisms such as the bacteria to exist in deep ocean without sunlight.

The Endeavour Hydrothermal Vents are a group of hydrothermal vents in the north-eastern Pacific Ocean, located 260 kilometres (160 mi) southwest of Vancouver Island, British Columbia, Canada. The vent field lies 2,250 metres (7,380 ft) below sea level on the northern Endeavour segment of the Juan de Fuca Ridge. In 1982, dredged sulfide samples were recovered from the area covered in small tube worms and prompted a return to the vent field in August 1984, where the active vent field was confirmed by HOV Alvin on leg 10 of cruise AII-112.
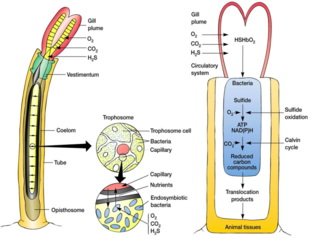
A trophosome is a highly vascularised organ found in some animals that houses symbiotic bacteria that provide food for their host. Trophosomes are contained by the coelom of tube worms and in the body of symbiotic flatworms of the genus Paracatenula.

Tevnia is a genus of giant tube worm in the family Siboglinidae, with only one species, Tevnia jerichonana, living in a unique deep-sea environment. These deep sea marine species survive in environments like hydrothermal vents. These vents give off gas and toxic chemicals with the addition of having superheated temperatures. The giant tube worm prefers environments such as these despite the harsh temperature and toxic sea water.

The hydrothermal vent microbial community includes all unicellular organisms that live and reproduce in a chemically distinct area around hydrothermal vents. These include organisms in the microbial mat, free floating cells, or bacteria in an endosymbiotic relationship with animals. Chemolithoautotrophic bacteria derive nutrients and energy from the geological activity at Hydrothermal vents to fix carbon into organic forms. Viruses are also a part of the hydrothermal vent microbial community and their influence on the microbial ecology in these ecosystems is a burgeoning field of research.

Peter R. Girguis is a professor in the department of Organismic and Evolutionary Biology at Harvard University, where he leads a lab that studies animals and microbes that live in extreme environments. He and his lab also develop novel underwater instruments such as underwater mass spectrometers. Girguis was the founder and Chief Technology Officer of Trophos Energy from 2010 to 2012, which focused on commercializing microbial fuel cell technologies. The company was bought by Teledyne Benthos in 2012. Girguis currently serves as a board member of the Ocean Exploration Trust and the Schmidt Marine Technology Partners.
Hydrogen sulfide chemosynthesis is a form of chemosynthesis which uses hydrogen sulfide. It is common in hydrothermal vent microbial communities Due to the lack of light in these environments this is predominant over photosynthesis

Lamellibrachia satsuma is a vestimentiferan tube worm that was discovered near a hydrothermal vent in Kagoshima Bay, Kagoshima at the depth of only 82 m (269 ft) the shallowest depth record for a vestimentiferan. Its symbiotic sulfur oxidizer bacteria have been characterised as ε-Proteobacteria and γ-Proteobacteria. Subspecies have been later found associated with cold seeps at Hatsushima in Sagami Bay and at the Daini Tenryu Knoll in the Nankai Trough with specimens obtained at up to 1,170 m (3,840 ft) depth.
Oligobrachia is a genus in the family Siboglinidae, commonly known as beard worms. These beard worms are typically found at spreading centers, hydrothermal vents, and undersea volcanoes. The siboglinidae are annelids which can be found buried in sediments. Beard worms do not necessarily exist at one specific part of the world's oceans, however, they are spread out all over the ocean floors as long as the surrounding environment is similar; these are known as metapopulations. Most commonly, these organisms are found at the bottom of the ocean floor, whether it be at a depth of roughly 25 meters or hundreds of meters. Oligobrachia can typically be found near hydrothermal vents and methane seeps. An important characteristic of this genus is that it lacks a mouth and gut. Therefore, it relies on symbiotic bacteria to provide the host organism with energy to survive. The majority of oligobrachia that have been observed have been found in the Arctic and other high-latitude areas of the world's oceans.
Frenulata, "beard worms", is a clade of Siboglinidae, "tube worms". They are one of four lineages with numerous species. They may be the most basal clade in the family. Despite being the first tube worms to be encountered and described, they remain the least studied group. This is because of their slender shape, they often get destroyed as a result of being caught as bycatch or poor preservation. They are found primarily in deep, muddy sediments, cold seeps, and anoxic firth sediments.


















