
A zoonosis or zoonotic disease is an infectious disease of humans caused by a pathogen that can jump from a non-human to a human and vice versa.

Severe acute respiratory syndrome (SARS) is a viral respiratory disease of zoonotic origin caused by the virus SARS-CoV-1, the first identified strain of the SARS-related coronavirus. The first known cases occurred in November 2002, and the syndrome caused the 2002–2004 SARS outbreak. In the 2010s, Chinese scientists traced the virus through the intermediary of Asian palm civets to cave-dwelling horseshoe bats in Xiyang Yi Ethnic Township, Yunnan.
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, three of which are unassigned to a subfamily, and 78 species.
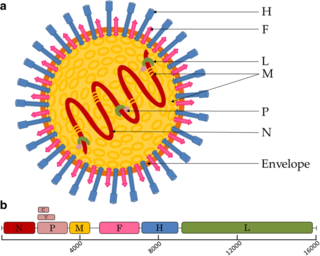
Henipavirus is a genus of negative-strand RNA viruses in the family Paramyxoviridae, order Mononegavirales containing six established species, and numerous others still under study. Henipaviruses are naturally harboured by several species of small mammals, notably pteropid fruit bats, microbats of several species, and shrews. Henipaviruses are characterised by long genomes and a wide host range. Their recent emergence as zoonotic pathogens capable of causing illness and death in domestic animals and humans is a cause of concern.

In infectious disease ecology and epidemiology, a natural reservoir, also known as a disease reservoir or a reservoir of infection, is the population of organisms or the specific environment in which an infectious pathogen naturally lives and reproduces, or upon which the pathogen primarily depends for its survival. A reservoir is usually a living host of a certain species, such as an animal or a plant, inside of which a pathogen survives, often without causing disease for the reservoir itself. By some definitions a reservoir may also be an environment external to an organism, such as a volume of contaminated air or water.

Nipah virus is a bat-borne, zoonotic virus that causes Nipah virus infection in humans and other animals, a disease with a very high mortality rate (40-75%). Numerous disease outbreaks caused by Nipah virus have occurred in South East Africa and Southeast Asia. Nipah virus belongs to the genus Henipavirus along with the Hendra virus, which has also caused disease outbreaks.

Severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), previously known as severe acute respiratory syndrome coronavirus (SARS-CoV), is a strain of coronavirus that causes severe acute respiratory syndrome (SARS), the respiratory illness responsible for the 2002–2004 SARS outbreak. It is an enveloped, positive-sense, single-stranded RNA virus that infects the epithelial cells within the lungs. The virus enters the host cell by binding to angiotensin-converting enzyme 2. It infects humans, bats, and palm civets. The SARS-CoV-1 outbreak was largely brought under control by simple public health measures. Testing people with symptoms, isolating and quarantining suspected cases, and restricting travel all had an effect. SARS-CoV-1 was most transmissible when patients were sick, so its spread could be effectively suppressed by isolating patients with symptoms.

Taiwan's epidemic of HIV/AIDS began with the first case reported in December 1984. On 17 December 1990 the government promulgated the AIDS Prevention and Control Act. On 11 July 2007, the AIDS Prevention and Control Act was renamed the HIV Infection Control and Patient Rights Protection Act.

The bat virome is the group of viruses associated with bats. Bats host a diverse array of viruses, including all seven types described by the Baltimore classification system: (I) double-stranded DNA viruses; (II) single-stranded DNA viruses; (III) double-stranded RNA viruses; (IV) positive-sense single-stranded RNA viruses; (V) negative-sense single-stranded RNA viruses; (VI) positive-sense single-stranded RNA viruses that replicate through a DNA intermediate; and (VII) double-stranded DNA viruses that replicate through a single-stranded RNA intermediate. The greatest share of bat-associated viruses identified as of 2020 are of type IV, in the family Coronaviridae.

A Nipah virus infection is a viral infection caused by the Nipah virus. Symptoms from infection vary from none to fever, cough, headache, shortness of breath, and confusion. This may worsen into a coma over a day or two, and 50 to 75% of those infected die. Complications can include inflammation of the brain and seizures following recovery.

The 1998–1999 Malaysia Nipah virus outbreak was a Nipah virus outbreak occurring from September 1998 to May 1999 in the states of Perak, Negeri Sembilan and Selangor in Malaysia. A total of 265 cases of acute encephalitis with 105 deaths caused by the virus were reported in the three states throughout the outbreak. The Malaysian health authorities at first thought that Japanese encephalitis (JE) was the cause of the infection. This misunderstanding hampered the deployment of effective measures to prevent the spread, before the disease was identified by a local virologist from the Faculty of Medicine, University of Malaya as a newly discovered agent. It was named Nipah virus (NiV). The disease was as deadly as the Ebola virus disease (EVD), but attacked the brain system instead of the blood vessels. University of Malaya's Faculty of Medicine and the University of Malaya Medical Centre played a major role in serving as a major referral centre for the outbreak, treating majority of the Nipah patients and was instrumental in isolating the novel virus and researched on its features.

Longan witches broom-associated virus is a species of positive-sense single-stranded RNA virus that has not been assigned to a genus within the family Potyviridae. It is thought to be the cause of witch's broom in longan, a large tropical tree from southeastern Asia of economic value. Longan witches broom disease is a condition that was first described in 1941. The virus was found in symptomatic plants and absent in healthy plants, but not all of Koch's postulates have been fulfilled.
Bat mumps orthorubulavirus, formerly Bat mumps rubulavirus (BMV), is a member of genus Orthorubulavirus, family Paramyxoviridae, and order Mononegavirales. Paramyxoviridae viruses were first isolated from bats using heminested PCR with degenerate primers. This process was then followed by Sanger sequencing. A specific location of this virus is not known because it was isolated from bats worldwide. Although multiple paramyxoviridae viruses have been isolated worldwide, BMV specifically has not been isolated thus far. However, BMV was detected in African fruit bats, but no infectious form has been isolated to date. It is known that BMV is transmitted through saliva in the respiratory system of bats. While the virus was considered its own species for a few years, phylogenetic analysis has since shown that it is a member of Mumps orthorubulavirus.
Ghanaian bat henipavirus (also known Kumasi virus belongs to the genus Henipavirus in the family Paramyxoviridae. Human infections are caused by zoonotic events where the virus crosses over from another animal species. Therefore, humans are not the innate host for this virus family but instead become infected by peripheral viral reservoirs such as bats and other carriers of the virus. When these virus are spread to humans through zoonotic events they have been found to be one of the most deadly viruses with the capability to infect humans, with mortality rates between 50 and 100%. Therefore, these viruses have been classified as a biosafety level four virus with regards to its pathogenesis when it infects humans.

This article documents the chronology and epidemiology of SARS-CoV-2 in January 2020, the virus which causes the coronavirus disease 2019 (COVID-19) and is responsible for the COVID-19 pandemic. The first human cases of COVID-19 were identified in Wuhan, China, in December 2019.

Chang Shan-chwen is a Taiwanese medical researcher and academic administrator. He convenes the advisory specialist panel of the Central Epidemic Command Center (CECC), which is associated with the Centers for Disease Control in Taiwan. Chang Shan-chwen is vice president of National Taiwan University and professor of medicine in the university's medical college.

This article documents the chronology and epidemiology of SARS-CoV-2 in 2019, the virus that causes coronavirus disease 2019 (COVID-19) and is responsible for the COVID-19 pandemic. The first human cases of COVID-19 known to have been identified were in Wuhan, Hubei, China, in December 2019. It marked the beginning of the 2019–2020 COVID-19 outbreak in mainland China.

Mòjiāng virus(MojV), officially Mojiang henipavirus, is a virus in the family Paramyxoviridae. Based on phylogenetics, Mòjiāng virus is placed in the genus Henipavirus or described as a henipa-like virus. Antibodies raised against Mòjiāng virus glycoproteins are serologically distinct from other henipaviruses (among which higher cross-reactivity is observed).

The COVID-19 pandemic in Beijing is part of the worldwide pandemic of the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease first reached Beijing on 20 January 2020.
A possibly ongoing outbreak of Langya henipavirus (LayV) was reported in China in August 2022, with 35 identified cases spanning from 2018 to August 2022. The index case was a 53-year-old female farmer who had been in contact with shrews and presented with a fever, headache, cough and nausea in Qingdao city. The virus was named "Langya" after the hometown of the index patient in Shandong.















