
Phomopsis cane and leaf spot occurs wherever grapes are grown. Phomopsis cane and leaf spot is more severe in grape-growing regions characterized by a humid temperate climate through the growing season. Crop losses up to 30% have been reported to be caused by Phomopsis cane and leaf spot.

Botryosphaeria dothidea is a plant pathogen that causes the formation of cankers on a wide variety of tree and shrub species. It has been reported on several hundred plant hosts and on all continents except Antarctica. B. dothidea was redefined in 2004, and some reports of its host range from prior to that time likely include species that have since been placed in another genus. Even so, B. dothidea has since been identified on a number of woody plants—including grape, mango, olive, eucalyptus, maple, and oak, among others—and is still expected to have a broad geographical distribution. While it is best known as a pathogen, the species has also been identified as an endophyte, existing in association with plant tissues on which disease symptoms were not observed. It can colonize some fruits, in addition to woody tissues.
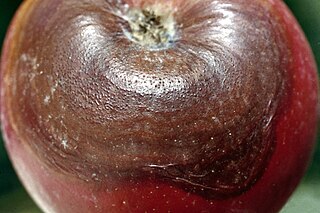
Botryosphaeria obtusa is a plant pathogen that causes frogeye leaf spot, black rot and cankers on many plant species. On the leaf it is referred to as frogeye leaf spot; this phase typically affects tree and shrubs. In fruit such as the apple, cranberry and quince, it is referred to as black rot, and in twigs and trunks it causes cankers.
Leptosphaeria coniothyrium is a plant pathogen. It can be found around the world.

Nectria cinnabarina, also known as coral spot, is a plant pathogen that causes cankers on broadleaf trees. This disease is polycyclic and infects trees in the cool temperate regions of the Northern Hemisphere. N. cinnabarina is typically saprophytic, but will act as a weak parasite if presented with an opportunity via wounds in the tree or other stressors that weaken the tree’s defense to the disease. A study published in 2011 showed that this complex consists of at least 4 distinct species. There are only a few ways to manage this disease with techniques such as sanitation and pruning away branches that have the cankers. N. cinnabarina is not as significant a problem as other Nectria spp., some of which are the most important pathogens to infect hardwood trees.
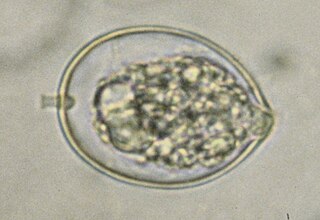
Phytophthora cactorum is a fungal-like plant pathogen belonging to the Oomycota phylum. It is the causal agent of root rot on rhododendron and many other species, as well as leather rot of strawberries.

Botryosphaeria ribis is a fungal plant pathogen that infects many trees causing cankers, dieback and death.

Diaporthe helianthi is a fungal pathogen that causes Phomopsis stem canker of sunflowers. In sunflowers, Phomopsis helianthi is the causative agent behind stem canker. Its primary symptom is the production of large canker lesions on the stems of sunflower plants. These lesions can eventually lead to lodging and plant death. This disease has been shown to be particularly devastating in southern and eastern regions of Europe, although it can also be found in the United States and Australia. While cultural control practices are the primary method of controlling for Stem Canker, there have been a few resistant cultivars developed in regions of Europe where the disease is most severe.

Didymella bryoniae, syn. Mycosphaerella melonis, is an ascomycete fungal plant pathogen that causes gummy stem blight on the family Cucurbitaceae, which includes cantaloupe, cucumber, muskmelon and watermelon plants. The anamorph/asexual stage for this fungus is called Phoma cucurbitacearum. When this pathogen infects the fruit of cucurbits it is called black rot.

Pyrenochaeta lycopersici is a fungal plant pathogen, infecting tomatoes and causing corky root rot.

Glomerella cingulata is a fungal plant pathogen, being the name of the sexual stage (teleomorph) while the more commonly referred to asexual stage (anamorph) is called Colletotrichum gloeosporioides. For most of this article the pathogen will be referred to as C. gloeosporioides. This pathogen is a significant problem worldwide, causing anthracnose and fruit rotting diseases on hundreds of economically important hosts.
Bot canker of oak is a disease on stems, branches and twigs of oak trees in Europe and North America. The casual agent of Bot canker of oak is the fungus Botryosphaeria corticola. Bot canker of oak causes lesions and cankers on a wide range of oaks in Europe and most recently live oaks in North America. Some infections were formerly attributed to Botryosphaeria stevensii, but most likely represent infections by Botryosphaeria corticola. Botryosphaeria corticola is distinguishable from Botryosphaeria stevensii via ITS rDNA sequencing.

Raspberry spur blight is caused by the fungus Didymella applanata. This plant pathogen is more problematic on red raspberries (Rubus idaeus) than on black or purple raspberries. The fungus infects the leaves first and then spreads to the cane. It causes necrotic spots on the cane near the base of the petiole attachment. Raspberry spur blight can cause a significant reduction in yield, fruit blight, premature leaf drop, and weak bud and cane growth. The magnitude of damage is not clearly understood in the United States, however, studies from Scotland suggest damage to the cane itself is limited. The disease has minor economic impacts by reducing leaves in the summer or killing buds. Major economic damage occurs if the disease manages to kill the entire cane. In the United States, this disease is found in Oregon and Washington.
Leucostoma canker is a fungal disease that can kill stone fruit. The disease is caused by the plant pathogens Leucostoma persoonii and Leucostoma cinctum (teleomorph) and Cytospora leucostoma and Cytospora cincta (anamorphs). The disease can have a variety of signs and symptoms depending on the part of the tree infected. One of the most lethal symptoms of the disease are the Leucostoma cankers. The severity of the Leucostoma cankers is dependent on the part of the plant infected. The fungus infects through injured, dying or dead tissues of the trees. Disease management can consist of cultural management practices such as pruning, late season fertilizers or chemical management through measures such as insect control. Leucostoma canker of stone fruit can cause significant economic losses due to reduced fruit production or disease management practices. It is one of the most important diseases of stone fruit tree all over the world.
Gummy stem blight is a cucurbit-rot disease caused by the fungal plant pathogen Didymella bryoniae. Gummy stem blight can affect a host at any stage of growth in its development and affects all parts of the host including leaves, stems and fruits. Symptoms generally consist of circular dark tan lesions that blight the leaf, water soaked leaves, stem cankers, and gummy brown ooze that exudes from cankers, giving it the name gummy stem blight. Gummy stem blight reduces yields of edible cucurbits by devastating the vines and leaves and rotting the fruits. There are various methods to control gummy stem blight, including use of treated seed, crop rotation, using preventative fungicides, eradication of diseased material, and deep plowing previous debris.
Grapevine trunk diseases (GTD) are the most destructive diseases of vineyards worldwide. Fungicides with the potential to control GTD have been banned in Europe and there are no highly effective treatments available. Action to develop new strategies to fight these diseases are needed.
The foamy bark canker is a disease affecting oak trees in California caused by the fungus Geosmithia pallida and spread by the Western oak bark beetle. This disease is only seen through the symbiosis of the bark beetles and the fungal pathogen. The bark beetles target oak trees and bore holes through the peridermal tissues, making tunnels within the phloem. The fungal spores are brought into these tunnels by the beetles and begin to colonize the damaged cells inside the tunnels. Symptoms of the developing fungus include wet discoloration seeping from the beetle entry holes as the fungus begins to consume phloem and likely other tissues. If bark is removed, necrosis of the phloem can be observed surrounding the entry hole(s). As the disease progresses, a reddish sap and foamy liquid oozes from entry holes, thus giving the disease the name foamy bark canker. Eventually, after the disease has progressed, the tree dies. This disease is important because of its detrimental effects on oak trees and its ability to spread to several new Californian counties in just a couple of years.
Thousands of plant diseases have been recorded throughout the world, many of these causing heavy crop losses. Early detection and accurate diagnosis is essential for the effective management of plant disease. Thus the first step in studying any disease is its timely detection of the diseased plant. Quick initial detection is largely based on the signs and symptoms of disease.

Neoscytalidium dimidiatum was first described in 1933 as Hendersonula toruloidea from diseased orchard trees in Egypt. Decades later, it was determined to be a causative agent of human dermatomycosis-like infections and foot infections predominantly in the tropical areas; however the fungus is considered to be widespread. A newer name, Scytalidium dimidiatum, was applied to synanamorph of Nattrassia mangiferae, otherwise known as Neofusicoccum mangiferae. Substantial confusion has arisen in the literature on this fungus resulting from the use of multiple different names including: Torula dimidiata, Scytalidium dimidiatum, Fusicoccum dimidiatum, and Hendersonula toruloidea.













