
Lithium citrate (Li3C6H5O7) is a chemical compound of lithium and citric acid that is used as a mood stabilizer in psychiatric treatment of manic states and bipolar disorder. There is extensive pharmacology of lithium, the active component of this salt.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed.

Bioremediation broadly refers to any process wherein a biological system, living or dead, is employed for removing environmental pollutants from air, water, soil, flue gasses, industrial effluents etc., in natural or artificial settings. The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment. In comparison to conventional physicochemical treatment methods bioremediation may offer considerable advantages as it aims to be sustainable, eco-friendly, cheap, and scalable.

Trisodium citrate has the chemical formula of Na3C6H5O7. It is sometimes referred to simply as "sodium citrate", though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor, and is a mild alkali.

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.
Assimilation is the process of absorption of vitamins, minerals, and other chemicals from food as part of the nutrition of an organism. In humans, this is always done with a chemical breakdown and physical breakdown .chemical alteration of substances in the bloodstream by the liver or cellular secretions. Although a few similar compounds can be absorbed in digestion bio assimilation, the bioavailability of many compounds is dictated by this second process since both the liver and cellular secretions can be very specific in their metabolic action. This second process is where the absorbed food reaches the cells via the liver.

Orphenadrine is an anticholinergic drug of the ethanolamine antihistamine class; it is closely related to diphenhydramine. It is a muscle relaxant that is used to treat muscle pain and to help with motor control in Parkinson's disease, but has largely been superseded by newer drugs. It is considered a dirty drug due to its multiple mechanisms of action in different pathways. It was discovered and developed in the 1940s.
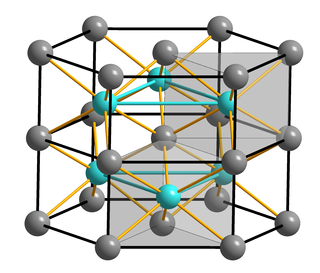
Iron(II) sulfide or ferrous sulfide is one of a family of chemical compounds and minerals with the approximate formula FeS. Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids.

Ammonium ferric citrate (also known as ferric ammonium citrate or ammoniacal ferrous citrate) has the formula (NH4)5[Fe(C6H4O7)2]. A distinguishing feature of this compound is that it is very soluble in water, in contrast to ferric citrate which is not very soluble.
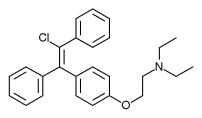
Enclomifene (INN), or enclomiphene (USAN), a nonsteroidal selective estrogen receptor modulator of the triphenylethylene group acts by antagonizing the estrogen receptor (ER) in the pituitary gland, which reduces negative feedback by estrogen on the hypothalamic-pituitary-gonadal axis, thereby increasing gonadotropin secretion and hence gonadal production of testosterone. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Enclomifene is the (E)-stereoisomer of clomifene, while zuclomifene is the (Z)-stereoisomer. Whereas zuclomifene is more estrogenic, enclomifene is more antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the ER and reduces testosterone levels in men. As such, isomerically pure enclomifene is more favorable than clomifene as a progonadotropin for the treatment of male hypogonadism.
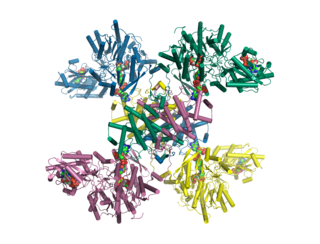
ATP citrate synthase (also ATP citrate lyase (ACLY)) is an enzyme that in animals represents an important step in fatty acid biosynthesis. By converting citrate to acetyl-CoA, the enzyme links carbohydrate metabolism, which yields citrate as an intermediate, with fatty acid biosynthesis, which consumes acetyl-CoA. In plants, ATP citrate lyase generates cytosolic acetyl-CoA precursors of thousands of specialized metabolites, including waxes, sterols, and polyketides.
Sodium citrate may refer to any of the sodium salts of citric acid :
Voges–Proskauer or VP is a test used to detect acetoin in a bacterial broth culture. The test is performed by adding alpha-naphthol and potassium hydroxide to the Voges-Proskauer broth, which is a glucose-phosphate broth that has been inoculated with bacteria. A cherry red color indicates a positive result, while a yellow-brown color indicates a negative result.

Ferrous citrate, also known as iron(II) citrate or iron(2+) citrate, describes coordination complexes containing citrate anions with Fe2+ formed in aqueous solution. Although a number of complexes are possible (or even likely), only one complex has been crystallized. That complex is the coordination polymer with the formula [Fe(H2O)6]2+{[Fe(C6H5O7)(H2O)]−}2.2H2O, where C6H5O73- is HOC(CH2CO2−)2(CO2−, i.e., the triple conjugate base of citric acid wherein the three carboxylic acid groups are ionized. Ferrous citrates are all paramagnetic, reflecting the weak crystal field of the carboxylate ligands.

Ferric citrate or iron(III) citrate describes any of several complexes formed upon binding any of the several conjugate bases derived from citric acid with ferric ions. Most of these complexes are orange or red-brown. They contain two or more Fe(III) centers.

Triammonium citrate is a chemical compound whose molecular formula is C6H17N3O7.

Aluminium citrate is a chemical compound with the chemical formula AlC
6H
5O
7. This white, crystalline salt is produced by mixing aluminium chloride hexahydrate and citric acid.
Alkali citrate is an inhibitor of kidney stones. It is used to increase urine citrate levels - this prevents calcium oxalate stones by binding to calcium and inhibiting its binding to oxalate. It is also used to increase urine pH - this prevents uric acid stones and cystine stones.

















