Related Research Articles

Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.
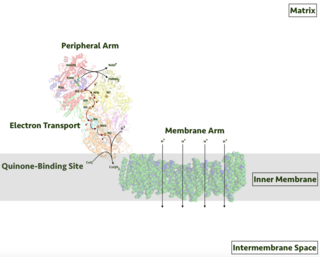
Respiratory complex I, EC 7.1.1.2 is the first large protein complex of the respiratory chains of many organisms from bacteria to humans. It catalyzes the transfer of electrons from NADH to coenzyme Q10 (CoQ10) and translocates protons across the inner mitochondrial membrane in eukaryotes or the plasma membrane of bacteria.

ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is:

Sir John Cowdery Kendrew, was an English biochemist, crystallographer, and science administrator. Kendrew shared the 1962 Nobel Prize in Chemistry with Max Perutz, for their work at the Cavendish Laboratory to investigate the structure of haem-containing proteins.

Sir John Ernest Walker is a British chemist who won the Nobel Prize in Chemistry in 1997. As of 2015 Walker is Emeritus Director and Professor at the MRC Mitochondrial Biology Unit in Cambridge, and a Fellow of Sidney Sussex College, Cambridge.

Richard Henderson is a British molecular biologist and biophysicist and pioneer in the field of electron microscopy of biological molecules. Henderson shared the Nobel Prize in Chemistry in 2017 with Jacques Dubochet and Joachim Frank.„Thanks to his work, we can look at individual atoms of living nature, thanks to cryo-electron microscopes we can see details without destroying samples, and for this he won the Nobel Prize in Chemistry."
Peter Nigel Tripp Unwin FRS is a British scientist at the MRC Laboratory of Molecular Biology, where he was Head of the Neurobiology Division from 1992 until 2008. He is currently also Emeritus Professor of Cell Biology at the Scripps Research Institute.

Modern biological research has revealed strong evidence that the enzymes of the mitochondrial respiratory chain assemble into larger, supramolecular structures called supercomplexes, instead of the traditional fluid model of discrete enzymes dispersed in the inner mitochondrial membrane. These supercomplexes are functionally active and necessary for forming stable respiratory complexes.

Christine Anne Orengo is a Professor of Bioinformatics at University College London (UCL) known for her work on protein structure, particularly the CATH database. Orengo serves as president of the International Society for Computational Biology (ISCB), the first woman to do so in the history of the society.

(Edith) Yvonne JonesFLSW is director of the Cancer Research UK Receptor Structure Research Group at the University of Oxford and a Fellow of Jesus College, Oxford. She is widely known for her research on the molecular biology of cell surface receptors and signalling complexes.

Jan Löwe is a German molecular and structural biologist and the Director of the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) in Cambridge, UK. He became Director of the MRC-LMB in April 2018, succeeding Sir Hugh Pelham. Löwe is known for his contributions to the current understanding of bacterial cytoskeletons.
Judy Hirst is a British scientist specialising in mitochondrial biology. She is Director of the MRC Mitochondrial Biology Unit at the University of Cambridge.

Lori Anne Passmore is a Canadian/British cryo electron microscopist and structural biologist who works at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) at the University of Cambridge. She is known for her work on multiprotein complexes involved in gene expression and development of new supports for cryo-EM.

Neil Alexander Steven Brockdorff is a Wellcome Trust Principal Research Fellow and professor in the department of biochemistry at the University of Oxford. Brockdorff's research investigates gene and genome regulation in mammalian development. His interests are in the molecular basis of X-inactivation, the process that evolved in mammals to equalise X chromosome gene expression levels in XX females relative to XY males.

Vassilis Pachnis Greek: Βασίλης Πάχνης is a Senior Group Leader in the Development and Homeostasis of the Nervous System Laboratory at the Francis Crick Institute.

Sjors Hendrik Willem ScheresFRS is a Dutch scientist at the MRC Laboratory of Molecular Biology Cambridge, UK.

Andrew P. Carter is a British structural biologist who works at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) in Cambridge, UK. He is known for his work on the microtubule motor dynein.

Kiyoshi Nagai was a Japanese structural biologist at the MRC Laboratory of Molecular Biology Cambridge, UK. He was known for his work on the mechanism of RNA splicing and structures of the spliceosome.
Caetano Maria Pacheco Pais dos Reis e Sousa is a senior group leader at the Francis Crick Institute and a professor of Immunology at Imperial College London.
Susan Mary Lea is a British biologist who serves as chief of the center for structural biology at the National Cancer Institute. Her research investigates host-pathogen interactions and biomolecular pathways. She was elected a Fellow of the Royal Society in 2022.
References
- 1 2 Leonid Sazanov publications indexed by Google Scholar
- ↑ Leonid Sazanov publications from Europe PubMed Central
- ↑ "IST Austria | Sazanov Group".
- 1 2 3 4 5 6 7 8 9 10 Anon (2019). "Professor Leonid Sazanov FRS". royalsociety.org. London: Royal Society. Archived from the original on 2019-04-24. One or more of the preceding sentences incorporates text from the royalsociety.org website where:
“All text published under the heading 'Biography' on Fellow profile pages is available under Creative Commons Attribution 4.0 International License.” --Royal Society Terms, conditions and policies at the Wayback Machine (archived 2016-11-11)
- ↑ Leonid Sazanov's ORCID 0000-0002-0977-7989
- ↑ Sazanov, L. A. (2006). "Structure of the Hydrophilic Domain of Respiratory Complex I from Thermus thermophilus". Science. 311 (5766): 1430–1436. Bibcode:2006Sci...311.1430S. doi: 10.1126/science.1123809 . ISSN 0036-8075. PMID 16469879. S2CID 1892332.

- ↑ Efremov, Rouslan G.; Baradaran, Rozbeh; Sazanov, Leonid A. (2010). "The architecture of respiratory complex I". Nature. 465 (7297): 441–445. Bibcode:2010Natur.465..441E. doi:10.1038/nature09066. ISSN 0028-0836. PMID 20505720. S2CID 4372778.

- ↑ Baradaran, Rozbeh; Berrisford, John M.; Minhas, Gurdeep S.; Sazanov, Leonid A. (2013). "Crystal structure of the entire respiratory complex I". Nature. 494 (7438): 443–448. Bibcode:2013Natur.494..443B. doi:10.1038/nature11871. ISSN 0028-0836. PMC 3672946 . PMID 23417064.
- ↑ Fiedorczuk, Karol (2017). Cryo-electron microscopy studies on ovine mitochondrial complex I. cam.ac.uk (PhD thesis). University of Cambridge. doi:10.17863/CAM.17168. OCLC 1063556549. EThOS uk.bl.ethos.744376.

- ↑ Burrows, P. A. (1998). "Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes". The EMBO Journal. 17 (4): 868–876. doi:10.1093/emboj/17.4.868. ISSN 1460-2075. PMC 1170436 . PMID 9463365.
- ↑ "Find people in the EMBO Communities". people.embo.org.