
Trematoda is a class of flatworms known as flukes or trematodes. They are obligate internal parasites with a complex life cycle requiring at least two hosts. The intermediate host, in which asexual reproduction occurs, is usually a snail. The definitive host, where the flukes sexually reproduce, is a vertebrate. Infection by trematodes can cause disease in all five traditional vertebrate classes: mammals, birds, amphibians, reptiles, and fish.

Digenea is a class of trematodes in the Platyhelminthes phylum, consisting of parasitic flatworms with a syncytial tegument and, usually, two suckers, one ventral and one oral. Adults commonly live within the digestive tract, but occur throughout the organ systems of all classes of vertebrates. Once thought to be related to the Monogenea, it is now recognised that they are closest to the Aspidogastrea and that the Monogenea are more closely allied with the Cestoda. Around 6,000 species have been described to date.

Trematodes are parasitic flatworms of the class Trematoda, specifically parasitic flukes with two suckers: one ventral and the other oral. Trematodes are covered by a tegument, that protects the organism from the environment by providing secretory and absorptive functions.

Echinostoma is a genus of trematodes (flukes), which can infect both humans and other animals. These intestinal flukes have a three-host life cycle with snails or other aquatic organisms as intermediate hosts, and a variety of animals, including humans, as their definitive hosts.

Leucochloridium is a genus of parasitic trematode worms in the order Diplostomida. It Is the sole genus in the family Leucochloridiidae. Members of this genus cause pulsating swellings in the eye-stalks of snails, so as to attract the attention of predatory birds required in the parasites' lifecycle.

Brachylaima is a genus of trematodes that can infect the gastrointestinal tract of human beings.

Succinea putris is a species of small air-breathing land snail in the family Succineidae, the amber snails.
Metagonimoides oregonensis is a trematode, or fluke worm, in the family Heterophyidae. This North American parasite is found primarily in the intestines of raccoons, American minks, frogs in the genus Rana, and freshwater snails in the genus Goniobasis. It was first described in 1931 by E. W. Price. The parasite has a large distribution, from Oregon to North Carolina. Adult flukes vary in host range and morphology dependent on the geographical location. This results in different life cycles, as well as intermediate hosts, across the United States. On the west coast, the intermediate host is freshwater snails (Goniobasis), while on the east coast the intermediate host is salamanders (Desmognathus). The parasites on the west coast are generally much larger than on the east coast. For example, the pharynx as well as the body of the parasite are distinctly larger in Oregon than in North Carolina. The reverse pattern is observed on the east coast for uterine eggs, which are larger on the west coast. In snails, there is also a higher rate of infection in female snails than in males. Research on the life history traits of the parasites have been performed with hamsters and frogs as model species.

Leucochloridium variae, the brown-banded broodsac, is a species of trematode whose life cycle involves the alternate parasitic infection of certain species of snail and bird. While there is no external evidence of the worm's existence within the bird host, the infection of the snail host is visible when its eye stalks become grotesquely engorged with the parasite's brood sacs. These brood sacks pulsate and move to imitate insect larva, attracting the parasite's next host, insectivore birds. The bird rips off the eye stalk and eats it, thus becoming infected. Later on, the parasite's eggs are dropped with the bird's feces. Similar life-histories are found in other species of the genus Leucochloridium, including Leucochloridium paradoxum.

Fasciolopsis is a genus of trematodes. They are also known as giant intestinal flukes.
Bucephalus polymorphus is a type of flatworm. This species is within the Bucephalidae family of Digenea, which in turn is a subclass of Trematodes within the phylum Platyhelminthes. It is characterized by having a mouth near the middle of its body, along with a sac-like gut. The mouth opening is located in the centre of the ventral surface. This is a specific body type of cecaria known as a gastrostome.
Halipegus eccentricus is a monoecious, digenea parasitic trematode commonly found in true frogs in North America. It was first described in 1939.

Echinostoma revolutum is a trematode parasite of which the adults can infect birds and mammals, including humans. In humans, it causes echinostomiasis.
Echinostoma hortense is an intestinal fluke of the class Trematoda, which has been found to infect humans in East Asian countries such as Korea, China, and Japan. This parasite resides in the intestines of birds, rats and other mammals such as humans. While human infections are very rare in other regions of the world, East Asian countries have reported human infections up to about 24% of the population in some endemic sub-regions. E. hortense infections are zoonotic infections, which occurs from eating raw or undercooked freshwater fish. The primary disease associated with an E. hortense infection is called echinostomiasis, which is a general name given to diseases caused by Trematodes of the genus Echinostoma.

Clinostomum marginatum is a species of parasitic fluke. It is commonly called the "yellow grub". It is found in many freshwater fish in North America, and no fish so far is immune to this parasite. It is also found in frogs. Clinostomum marginatum can also be found in the mouth of aquatic birds such as herons and egrets. They are commonly present in the esophagus of fish-eating birds and reptiles. Eggs of these trematodes are shed in the feces of aquatic birds and released into water. Aquatic birds become hosts of this parasite by ingesting infected freshwater fish. The metacercariae are found right beneath the skin or in the muscles of host fish.
Megalodiscus temperatus is a Digenean in the phylum Platyhelminthes. This parasite belongs to the Cladorchiidae family and is a common parasite located in the urinary bladder and rectum of frogs. The primary host is frogs and the intermediate hosts of Megalodiscus temeperatus are freshwater snails in the genus Helisoma.
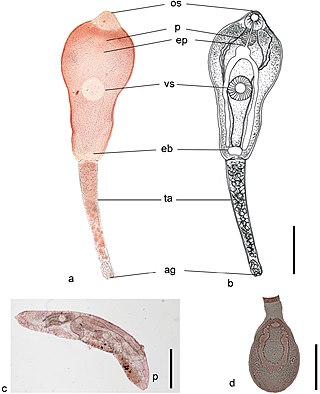
Philophthalmus gralli, commonly known as the Oriental avian eye fluke, parasitises the conjunctival sac of the eyes of many species of birds, including birds of the orders Galliformes and Anseriformes. In Brazil this parasite was reported in native Anseriformes species. It was first discovered by Mathis and Leger in 1910 in domestic chickens from Hanoi, Vietnam. Birds are definitive hosts and freshwater snail species are intermediate hosts. Human cases of philophthalmosis are rare, but have been previously reported in Europe, Asia, and America.
Microphallus turgidus is a widespread and locally common flatworm parasite in New Zealand lakes and streams. Multilocus allozyme genotype data show that Microphallus turgidus is a single outbred species with high levels of gene flow among South Island populations. Microphallus turgidus is commonly found in the abdominal muscles of grass shrimp.

Metagonimus yokogawai, or the Yokogawa fluke, is a species of a trematode, or fluke worm, in the family Heterophyidae.

Gastropod-borne parasitic diseases (GPDs) are a group of infectious diseases that require a gastropod species to serve as an intermediate host for a parasitic organism that can infect humans upon ingesting the parasite or coming into contact with contaminated water sources. These diseases can cause a range of symptoms, from mild discomfort to severe, life-threatening conditions, with them being prevalent in many parts of the world, particularly in developing regions. Preventive measures such as proper sanitation and hygiene practices, avoiding contact with infected gastropods and cooking or boiling food properly can help to reduce the risk of these diseases.



















