
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).

The areca nut is the fruit of the areca palm, which grows in much of the tropical Pacific, South Asia, Southeast Asia, and parts of east Africa. It is commonly referred to as betel nut, not to be confused with betel leaves that are often used to wrap it. Consumption has many harmful effects on health and is carcinogenic to humans. Various compounds present in the nut, including arecoline, contribute to histologic changes in the oral mucosa. It is known to be a major risk factor for cancers of the mouth and esophagus. As with chewing tobacco, its use is discouraged by preventive efforts. Consumption by hundreds of millions of people worldwide – mainly of South Asian or Southeast Asian origins – has been described as a "neglected global public health emergency".

Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

Isopentenyl pyrophosphate is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
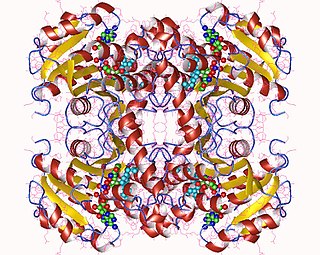
Enoyl-acyl carrier protein reductase, is a key enzyme of the type II fatty acid synthesis (FAS) system. ENR is an attractive target for narrow-spectrum antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species. In addition, the bacterial ENR sequence and structural organization are distinctly different from those of mammalian fatty acid biosynthesis enzymes.
In enzymology, a dihydrokaempferol 4-reductase (EC 1.1.1.219) is an enzyme that catalyzes the chemical reaction
In enzymology, a retinol dehydrogenase (RDH) (EC 1.1.1.105) is an enzyme that catalyzes the chemical reaction

In enzymology, a 3-oxoacyl-[acyl-carrier-protein] reductase (EC 1.1.1.100) is an enzyme that catalyzes the chemical reaction
In enzymology, a leucocyanidin oxygenase (EC 1.14.11.19) is an enzyme that catalyzes the chemical reaction
In enzymology, a leucoanthocyanidin reductase (EC 1.17.1.3) (LAR, aka leucocyanidin reductase or LCR) is an enzyme that catalyzes the chemical reaction

Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

Aromadendrin is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.
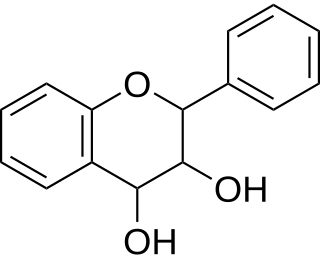
Leucoanthocyanidin (flavan-3,4-diols) are colorless chemical compounds related to anthocyanidins and anthocyanins. Leucoanthocyanins can be found in Anadenanthera peregrina and in several species of Nepenthes including N. burbidgeae, N. muluensis, N. rajah, N. tentaculata, and N. × alisaputrana.

Leucocyanidin is a colorless chemical compound that is a member of the class of natural products known as leucoanthocyanidins.
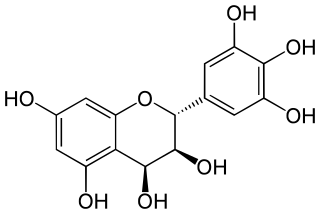
Leucodelphinidin is a colorless chemical compound related to leucoanthocyanidins. It can be found in Acacia auriculiformis, in the bark of Karada and in the kino (gum) from Eucalyptus pilularis.
The biosynthesis of phenylpropanoids involves a number of enzymes.














